Table 8-2 Equivalent conductivityλfor cations at infinite dilution


Photo 8-1 Electric conductivity meter
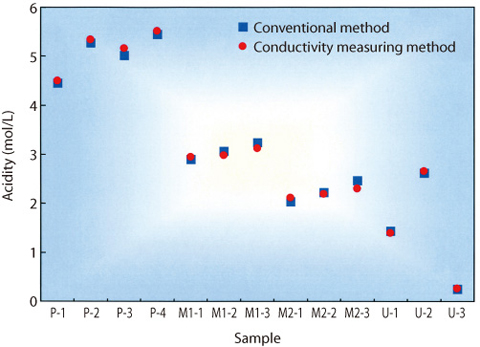
Fig.8-7 Comparison of results of electric conductivity measuring method and conventional method
Acidity (hydrogen ion:H+) in nitric acid solutions containing plutonium(Pu) and uranium(U) at high concentration handled in reprocessing plant is an important parameter for control of the reprocessing processes, specifically to prevent the hydrolysis of Pu in the processes.
Potentiometric titration method has been applied to determine the acidity in these solutions as conventional method. In this method, it is necessary to add reagents such as sodium hydroxide (NaOH), including fluoride ion (F-), and they become waste liquid. Particularly, as it is known that F- accelerates corrosion of stainless steel at room temperature, the corrosion of tanks and pipes for treatment of the waste liquid has been feared. Thus, we have searched for an analytical method that measures the acidity without using reagents including F- and is rapid.
We focused on the electric conductivity of these solutions. Electric conductivity shows ease of current flow, and depends on the temperature of the solution, and on the variety and the concentration of ions (electrolytes) contained in the solution. Particularly, equivalent conductivity of H+ is very high in comparison with that of other cations shown in Table 8-2, and so can be measured with high sensitivity. Furthermore, electric conductivity can be easily measured by a commercial electric conductivity meter without any improvements, as shown in Photo 8-1. However, when various ions exist in the solution, electric conductivity of the solution represents sum of electric conductivities from all the ions. Consequently, it is impossible to measure only electric conductivity of H+, when Pu and U exist in solutions like Pu-U mixed nitrate solution at high concentration. Therefore, using the correlation between electric conductivity and concentration of H+ in the solutions, we have investigated an analytical method that is able to determine H+ in the solutions containing Pu and U by measuring electric conductivity of a sample diluted with distilled water at constant temperature (25°C), and correlating the electric conductivity and acidity using a multivariate analysis method. As a result, we obtained good results as follows,
(1) Acidity in the nitric acid solutions containing Pu and/or U obtained by this method was in good agreement with that of the conventional method, within 10%, as shown in Fig.8-7.
(2) For Pu nitrate solution and Pu-U mixed nitrate solution, the repeatability of the measurement of electric conductivity at 25.0°C was within 1.5%.
(3) It takes about 5 min to analyze by this method, 1/6 the time of the conventional method.
From the results described above, an electric conductivity measuring method can be applied to analysis of acidity in the nitric acid solutions containing Pu and U at the high concentration handled in the Plutonium Conversion Development Facility. Furthermore, this method should be applicable to analysis of acidity in the nitric acid solutions containing Pu and U use in reprocessing process.
<Previous: 8-4 | Next: 9 Development of Technology on Nuclear Cycle Backend >