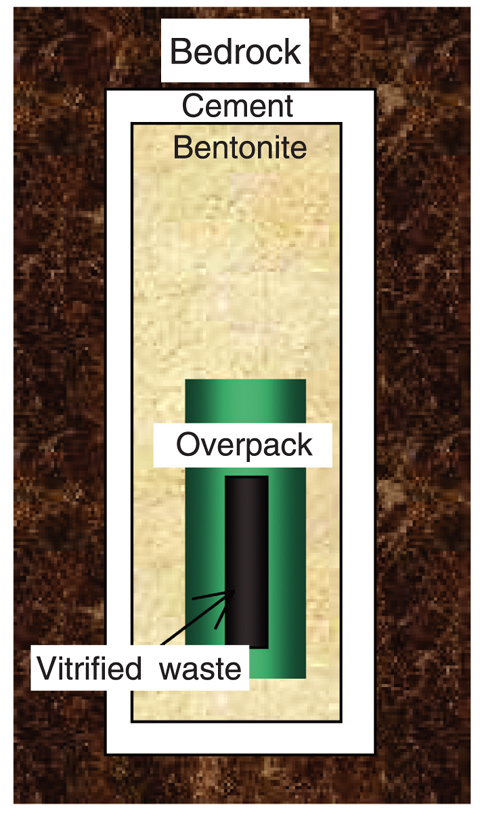
Fig.5-17 A conceptual view of high-level radioactive waste (HLW) disposal system
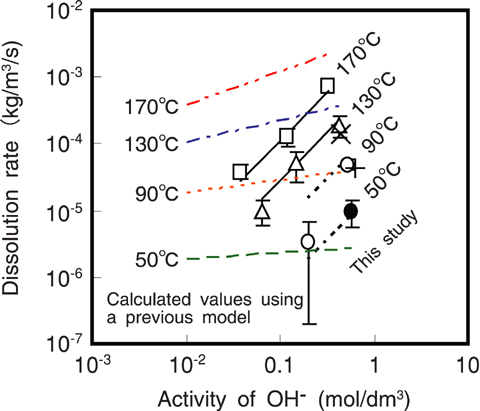
Fig.5-18 Dissolution rate of montmorillonite in highly alkaline solutions
In the Japanese program, high-level radioactive waste (HLW) is vitrified, encapsulated in a metal container called overpack, surrounded by engineered bentonite buffer material, and placed in a repository cut out from stable bedrock as shown in Fig.5-17. Cement is expected to be applied for the mechanical support of the repository.
Besides its mechanical buffer function, bentonite prevents groundwater from intruding into the waste and retards radionuclide movement outwards by adsorbing them. It is necessary to estimate the long-term changes of such properties of bentonite confining long-lived radionuclides in HLW.
Radioactive decay of radionuclides in HLW increases the temperature of the surrounding bentonite up to 90°C right after the emplacement, and an alkaline environment is likely to be induced by the cement used in the repository. The temperature and alkaline condition possibly accelerate alteration of bentonite.
Because the radionuclide confining property of the bentonite arises from its main constituent montmorillonite, dissolution of montmorillonite in contacting groundwater results in deterioration of the confinement. Several researchers have obtained the dissolution rates of pure pulverized montmorillonite in free water with high water/solid-ratio, which are far from the repository condition where bentonite is in a compacted state. The property of water filling the narrow pore spaces (porewater) in the compacted bentonite may be different from that of free water. We performed the dissolution experiments of montmorillonite in a compacted state simulating the repository condition.
The dissolution rate of montmorillonite we obtained was a function of activity of hydroxide ions, aOH- (mol/dm3), and temperature, T (K), as shown in Fig.5-18. The rate, RA (kg/m3/s), could be formulated as:
RA = 3.5 × 103(aOH-)1.4exp(-51000/RT)[kg/m3/s]
where R is the gas constant. The dissolution rate obtained for a compacted state has a higher dependence on the aOH- than that of previous models that were based on high water/solid-ratio dissolution experiments using pulverized materials.
The aOH- of porewater is an important factor determining the dissolution rate, and this is determined by both chemical reactions with minerals and mass transport. We developed a coupled mass-transport/ chemical-reaction code for predicting the aOH- and the long-term alteration of the bentonite.
Further investigation is necessary to understand the mechanisms of the dissolution of compacted montmorillonite and to verify the model by comparing results of the model calculation with laboratory experiments or field observations.