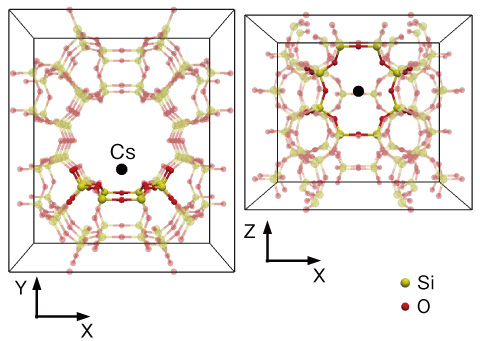
Fig.1-40 Crystal structure of zeolite called mordenite
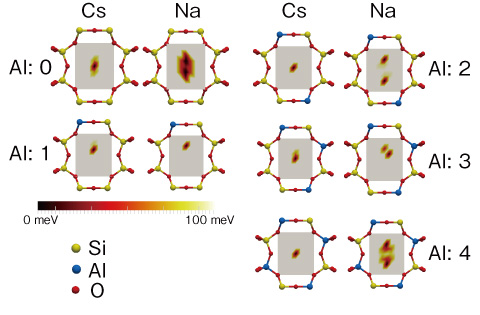
Fig.1-41 Absorption energy distribution at Cs+ absorption site
After the accident at the TEPCO’s Fukushima Daiichi NPS, the treatment of nuclear waste water, which includes radionuclides such as radioactive cesium, became an important problem. Cesium is presently removed from waste water by absorbent materials, for example, zeolite. Although more than 100 types of zeolite have been discovered so far, some of them absorb cesium well, and some absorb very little. If we develop a new zeolite that absorbs more cesium and has better controllability of absorption and removal, treatment of waste water will advance.
To develop desirable zeolites, we have to know how zeolites absorb cesium. However, it is difficult to directly observe the absorption behavior of cesium because it occurs at the microscopic scale. In this case, numerical simulations at the atomic scale are effective for revealing the absorption mechanism.
The characteristics of zeolites that absorb Cs selectively are known empirically. However, the principles of the selectivity mechanism have been unclear. To clarify these principles, we calculate in detail the absorption energy at the Cs absorption site in mordenite (Fig.1-40), which is a zeolite with a high Cs-selective absorbability. We adopted the first-principles calculation method to evaluate the absorption energy because it is the most accurate and reliable. The results are shown in Fig.1-41. This figure shows density plots of the absorption energy of Cs and sodium (Na). For Na, the minimum points are distributed, and therefore a Na ion is weakly bound at the site. On the other hand, a Cs ion is strongly bound at only one minimum-energy point in the center of the site. Thus, we determined why Cs is bound more strongly than Na at this site. By numerical simulations, we also identified various characteristics of Cs-selective absorption, some of which were previously unknown.
With this deeper understanding of the absorption mechanism, we can appropriately plan the development of high-performing absorbent materials. This development can contribute to not only treatment of waste water but also decontamination of environmental radionuclides.