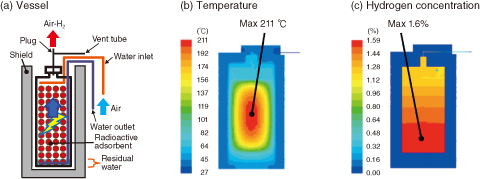
Fig.1-39 An example of the thermal hydraulic analysis of a Cs adsorption vessel with residual water
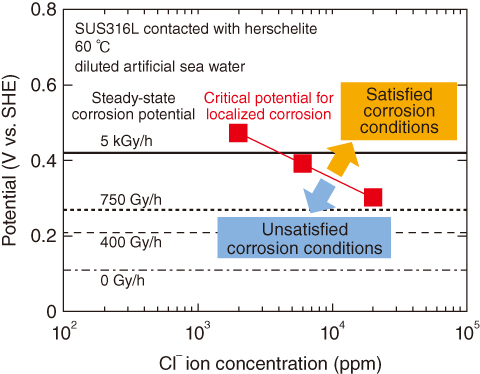
Fig.1-40 Electrochemical corrosion tests of stainless steel SUS316L
Stainless steel vessels containing mineral adsorbents (herschelite, etc.), which possess high selectivity toward cesium (Cs), are used for the treatment of radioactive saline water at the accident at the TEPCO’s Fukushima Daiichi NPS. The spent vessel is highly radioactive and temporarily stored with the shield (Fig.1-39(a)). For long-term storage, we evaluated the hydrogen production and corrosion of herschelite adsorption vessels.
As fundamental properties of herschelite, we investigated its thermal conductivity, hydrogen production yield, and Cs adsorption coefficients, as well as determined the Cs activity, decay heat, absorbed dose rate, and hydrogen production rate. The hydrogen concentration in a reference vessel of uniform Cs distribution was evaluated based on these fundamental properties. Fig.1-39(b) shows that the maximum temperature is 211 ℃, below the self-ignition temperature of hydrogen (500-571 ℃). This thermal hydraulic behavior maintains the maximum hydrogen concentration below 1.6% (Fig.1-39(c)) and well below the lower explosive limit of 4%.
An electrochemical method was employed to determine the immunity to localized corrosion. Fig.1-40 shows the potentials of SUS316L in a mixture of herschelite and sea water. The critical potential for localized corrosion decreased as the Cl– ion concentration increased. The conditions for localized corrosion are satisfied when the critical potential is lower than the steady-state corrosion potential. Therefore, under the conditions (temperature < 60 ℃, adsorbed dose rate of 755 Gy/h) shown in Fig.1-39, the localized corrosion of SUS316L in contact with herschelite would not immediately occur at Cl– ion concentrations of 20000 ppm.
Part of this study was the results of research entrusted to JAEA by the Agency for Natural Resources and Energy, Ministry of Economy, Trade and Industry of Japan (METI).