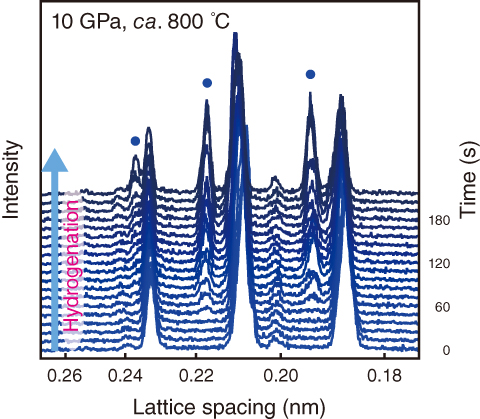
Fig.5-17 In situ synchrotron radiation X-ray diffraction profiles of hydrogenated Al2Cu
 ) indicate the Bragg peaks from Al2CuH.
) indicate the Bragg peaks from Al2CuH.
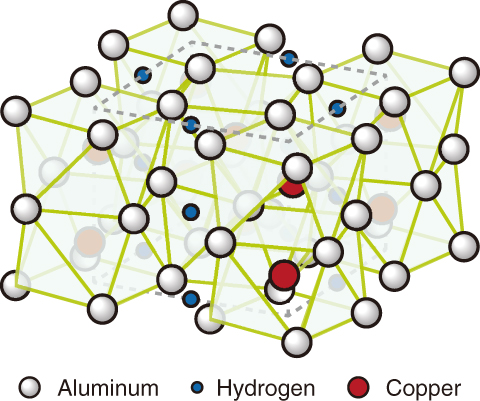
Fig.5-18 Crystal structure of Al2CuH
Developing safe and efficient hydrogen storage is among the primary technological challenges to realizing a hydrogen-based economy. Lightweight hydrogen storage materials are required for automotive applications. Aluminum is a suitable material because it is lightweight, harmless, and abundant in nature. However, although complex aluminum hydrides have been extensively investigated, they have not been considered for hydrogen storage applications. Apart from complex aluminum hydrides, few aluminum-based interstitial hydrides have been synthesized to date, although interstitial hydride-referred to as a “hydrogen-storage alloy”-exhibits excellent hydrogen reversibility.
We synthesized an aluminum-based interstitial hydride by hydrogenating a powdered aluminum-copper alloy (Al2Cu) at high pressure and temperature. Under these conditions, hydrogen becomes extremely reactive. High-pressure and high-temperature conditions were generated by a cubic-type multi-anvil apparatus. The hydrogenation conditions were explored by an In situ synchrotron radiation X-ray diffraction measurement system installed on the BL14B1 at SPring-8.
Fig.5-17 shows the X-ray diffraction profiles of Al2Cu hydrogenated at 10 GPa. After 60 s incubation at ca. 800 ℃, new Bragg peaks ( ) began to appear in the profile. The appearance of the peaks indicated that Al2Cu was hydrogenated to Al2CuH. The formed hydride was recovered at ambient conditions, and its crystal structure was characterized by a powder X-ray diffractometer. The obtained crystal structure of Al2CuH (Fig.5-18) is consistent with the formation of an aluminum-based interstitial hydride. This structure is further supported by first-principles calculations.
) began to appear in the profile. The appearance of the peaks indicated that Al2Cu was hydrogenated to Al2CuH. The formed hydride was recovered at ambient conditions, and its crystal structure was characterized by a powder X-ray diffractometer. The obtained crystal structure of Al2CuH (Fig.5-18) is consistent with the formation of an aluminum-based interstitial hydride. This structure is further supported by first-principles calculations.
We conclude that an aluminum-based interstitial hydride, Al2CuH, was successfully synthesized. The experimental and theoretical results of this study will assist the exploration of other aluminum-based interstitial hydrides, and the development of practical lightweight hydrogen storage materials.
The present study was partly sponsored by New Energy and Industrial Technology Development Organization (NEDO), and by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research (Nos. 25220911, 25420725).