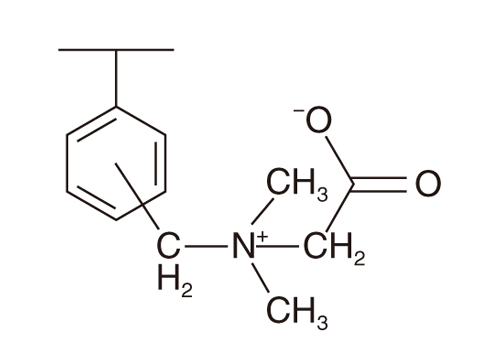
Fig.4-24 Schematic formula of betaine resin, AMP03
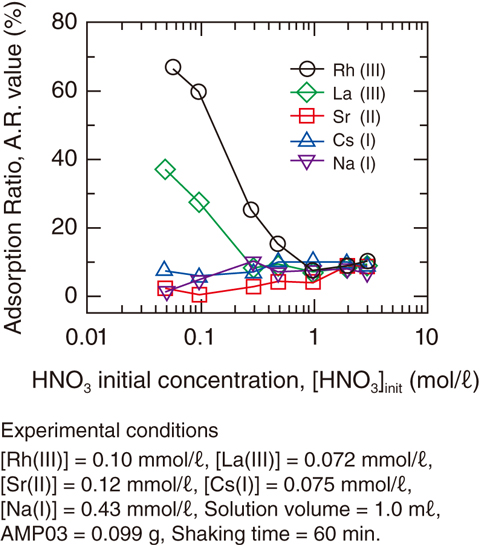
Fig.4-25 Adsorption behavior of metal ions with AMP03
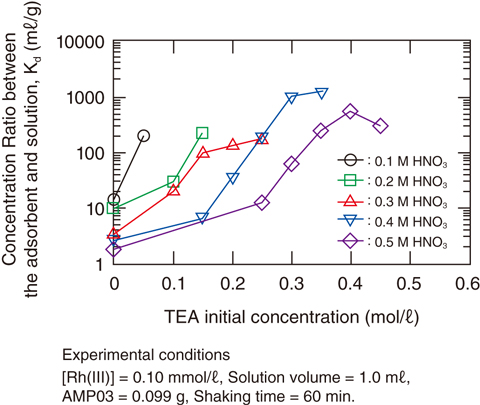
Fig.4-26 Adsorption behavior of Rh(III) with AMP03
High-level radioactive waste (HLW) from reprocessing process contains various elements. Some of these are valuable, such as rare metals. If the valuable rare metal in HLW is recovered for industrial usage, it may become an important resource. Rhodium (Rh) is a platinum-group metal that is necessary for the production of catalytic agents used in automobiles. The price of Rh is relatively high and will increase further with growing automobile demand. Relatively large amounts of Rh are generated as fission products. Although radioactive 102Rh and 102mRh, with half-lives of 2.9 and 0.57 years, respectively, are present in minor amounts, almost all Rh generated in fission reactors is of the stable isotope 103Rh. Therefore, after suitable storage, Rh can be used in industrial applications.
We examined Rh(III) adsorption using styrenedivinylbenzene copolymer functionalized with betaine (AMP03, Fig.4-24). AMP03 is generally used to separate sugar groups and salts. Consequently, AMP03 was found to successfully adsorb Rh(III) from the HNO3 solution with pH 1.72. AMP03 is composed of C, H, O, and N and is favorable for reducing secondary radioactive waste. This point is advantageous for application to Rh(III) recovery from HLLW.
The adsorption of Rh(III), La(III), Sr(II), Cs(I), and Na(I) from an HNO3 solution was also studied by varying the HNO3 concentration in the initial solution, [HNO3]init. The results are shown in Fig.4-25;Rh(III) and La(III) were adsorbed in the low-[HNO3]init range. The adsorption-ratio (AR) values of Rh(III) and La(III) decreased with increasing [HNO3]init. By contrast, AMP03 showed lower affinity for Sr(II), Cs(I), and Na(I) across the entire range of [HNO3]init values (AR < 10%). The order of the adsorption selectivity for AMP03 (Rh(III) > La(III) > Cs(I), Sr(II), Na(I)) is advantageous for the selective recovery of Rh(III).
The Kd values of Rh(III) for AMP03 greatly increased with the addition of trimethylamine (TEA). To obtain detailed data for the efficient recovery of Rh(III), adsorption experiments were performed using 0.1–0.5M HNO3 solution containing Rh(III) and TEA. Fig.4-26 shows plots of Kd against the concentration of TEA contained in the initial solutions [TEA]init. All Kd values in Fig.4-26 significantly increased with the addition of the amine compound. Effective Rh(III) adsorption with Kd > 100 was also observed.
R&D activities aimed at establishing reasonable treatment processes for HLW will be conducted.