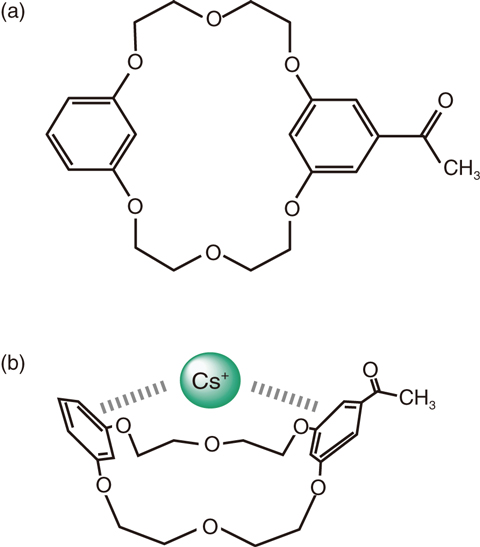
Fig.5-18 Coordination structure composed of DB20C6 and Cs+
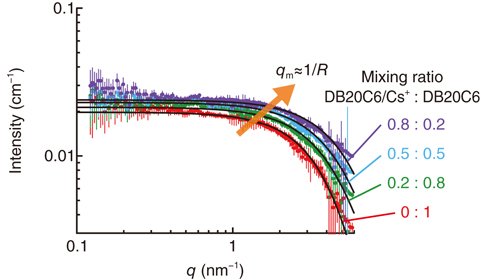
Fig.5-19 SANS profiles obtained for the mixtures of DB20C6 and DB20C6/Cs+
Following the accident at the TEPCO’s Fukushima Daiichi NPS in Japan in 2011, radioactive cesium was released into the northern Kanto and southern Tohoku areas. The development of physicochemical techniques for removing cesium ions (Cs+) is required to decrease the radioactivity in these areas. In particular, the selective separation of Cs+ from other cations contributes to remediation and reduction of the volume of contamination. Crown ethers are macrocyclic organic compounds that exhibit electrostatic interactions between an alkali-metal ion and multiple oxygen atoms in the ether ring and are thus difficult to use for selective separation of Cs+. Against this background, we found a derivative of benzo-crown ether, mono-acetyl substituted dibenzo-20-crown-6-ether (DB20C6) (Fig.5-18(a)), which preferentially forms stable complexes with Cs+ as compared with Na+ and K+. Understanding the selectivity mechanism contributes to the development of more effective adsorbents for Cs+ in solution.
In this study, we used density-functional-theory (DFT) calculation and small-angle neutron scattering (SANS) to elucidate the mechanism of this selectivity. The result of DFT calculation suggested that the stability of the complex formed by DB20C6 and Cs+ (DB20C6/Cs+) arose from the interaction between the π-orbitals of the two benzene rings and the d-orbital of Cs+. That is, the π-electrons of the benzene rings are donated to the vacant d-orbitals. This calculation indicates that Cs+ is stably bound above the center of the ether-oxygen ring by the two benzene rings and that DB20C6 adopts the folded structure shown in Fig.5-18(b). Note that neither Na+ nor K+ can form the π–d hybrid orbital because there are no electrons in their d-orbitals. Therefore, hybridization would be attributed to the high selectivity of Cs+ among alkali metal ions.
Fig.5-19 shows the SANS profiles obtained for mixtures of DB20C6/Cs+ and free DB20C6 in deuterated dimethyl sulfoxide (DMSO-d6) as functions of their mixing ratios. The characteristic q position, which starts to decrease in small-angle scattering intensity at this position, moves toward higher q as the fraction of DB20C6/Cs+ in the solution increases, indicating that the size of DB20C6/Cs+ is smaller than that of free DB20C6 in accordance with the coordination structure predicted by DFT calculation. The experimental data agreed well with SANS profiles calculated using the Debye function for scattering on an absolute scattering-intensity scale. Here, the structures of DB20C6/Cs+ and free DB20C6 from the DFT calculation were used for this theoretical calculation of the scattering profiles. This result leads us to the conclusion that SANS analysis experimentally supports the coordination structure of DB20C6/Cs+ and lends credence to the prediction that the driving force behind Cs+ selective coordination is hybridization between the π electrons of the two benzene rings and the d-orbital of Cs+. This work will contribute to the further development of materials for separating radioactive Cs+ in solution.