Fig.9-6 Atomic motions in a crystal of thorium dioxide
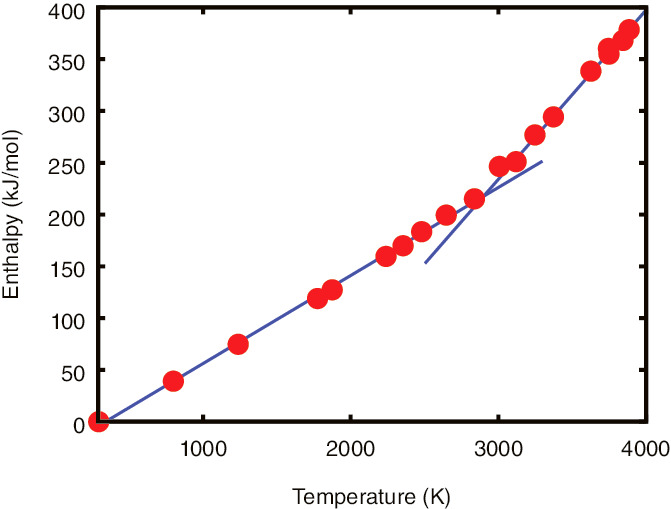
Fig.9-7 Temperature dependence of the enthalpy of thorium dioxide
In developing safer and more efficient nuclear fuels, it is crucial to understand their high-temperature properties. However, it is not easy to reproduce extreme situations inside nuclear reactors in laboratories for the purposes of measuring various nuclear-fuel properties. Thus, it is expected that numerical simulations will help experimenters to evaluate the thermophysical properties of fuels.
While thorium dioxide is a candidate next-generation fuel, its high-temperature properties, which are important in severe-accident analysis, have yet to be investigated in detail. In most actinide dioxides, anomalous behaviors are known to be observed at high temperature slightly below their melting point. For example, the enthalpy of uranium dioxide increases drastically above 2500 K, whereas it increases slowly below 2500 K. Though similar phenomena have been predicted in the case of thorium dioxide, the drastic change in the enthalpy increment has not been observed clearly due to the low accuracy of the experiments.
We explored the high-temperature behavior of thorium dioxide using first-principles molecular-dynamics simulations. Such simulation is a method to analyze the motions of atoms with atomic forces calculated from the fundamental interactions of nuclei and electrons. According to this method, high-accuracy calculations are possible without empirical parameters. We evaluated the relationship between the enthalpy of thorium dioxide and temperature by numerical simulations using supercomputers, where we prepared a calculation cell of a thorium-dioxide crystal with 324 atoms (Fig.9-6). As shown in Fig.9-7, we discovered a drastic increment of enthalpy above 3000 K; the calculated transition temperature of thorium dioxide is 500-K larger than that of uranium dioxide. We found that the drastic changes in enthalpy were blurred in experiments, though this was clear in our calculations due to their high accuracy. Moreover, we also found that, above 3000 K, oxygen atoms moved almost freely as atoms in liquid do. These motions were revealed to cause drastic changes in enthalpy.
Thus, numerical simulations enable us to evaluate various properties of materials, some of which are difficult to measure by experiment. By doing so, we hope to contribute to the development of safer nuclear fuels.