Fig.5-19 Chemical formula (left) and structure (right) of the developed BIZA
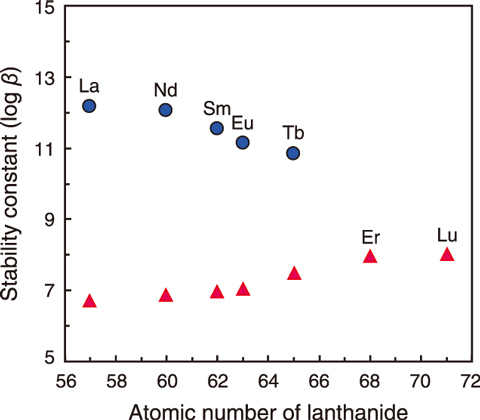
Fig.5-20 Complexation ability of BIZA with lanthanide
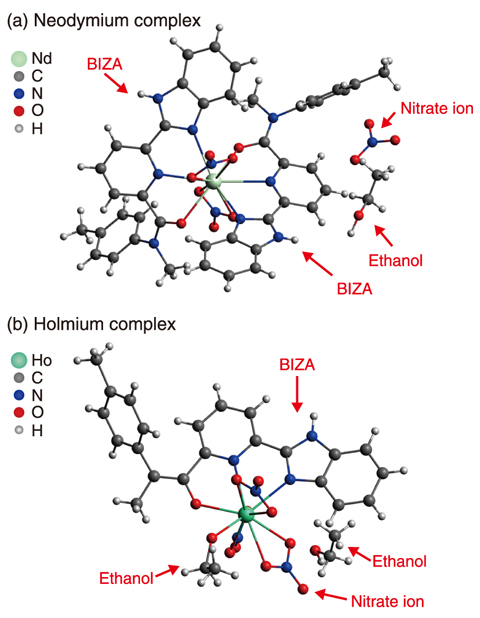
Fig.5-21 Crystal structure of lanthanide complex of BIZA
Lanthanides (Lns) are essential for high-technology products, such as the voice coil motor of an electric vehicle or a hard disk drive. As Japan’s manufacturing industry is currently highly dependent on Ln imports, the establishment of an efficient separation and recovery technique from waste products would help stabilize the supply. However, separating Lns is very difficult due to their similarities in chemical properties. During ongoing efforts to develop techniques to efficiently separate specific Lns, the compound BIZA, shown in Fig.5-19, was found to recognize slight differences in the ionic size of Lns.
BIZA demonstrated a characteristic complexation with Ln, as shown in Fig.5-20, investigated by spectroscopic titration. BIZA formed two complex types: one Ln ion with one BIZA molecule (1:1 complex) and one Ln ion with two BIZA molecules (1:2 complex). The 1:1 complex became easier to form with an increased atomic number of the Ln. This behavior has often been shown in compounds that form complexes with Ln. However, forming the 1:2 complex became more difficult with an increasing atomic number; this complex did form after dysprosium (Dy), which has an atomic number of 66.
The detailed structures of these complexes were thus investigated by X-ray crystallography to further understand the mechanisms behind this behavior. The crystal structures of a Neodymium (Nd) complex (atomic number 60) and a Holmium (Ho) complex (atomic number 67) are shown as examples in Fig.5-21. Of the several crystal samples examined, all Nd formed a 1:2 complex and all Ho formed a 1:1 complex. In both the Nd and Ho complexes, BIZA coordinates with Ln as tridentate ligand through the oxygen atom in the amide group and nitrogen atom in the pyridyl and imidazole groups. The coordination bond distance was longer in the 1:2 complex than in the 1:1 complex and, among the 1:2 complexes, increased with increasing atomic number of Ln. As the atomic number increased, the ionic size of Ln slightly and gradually decreased, causing the coordinated molecules to become closer. However, for bulky molecules such as BIZA, repulsion between molecules occurs when they are too close together. As a result, in the 1:2 complex, the coordination bond became weaker with increasing the atomic number; past an atomic number of 65, two BIZA molecules could not coordinate with one Ln ion. Thus, the relationship between the bulkiness of ligand molecule and the ionic size of Ln determines the limit element which can form stable 1:2 complexes.
The ionic size suitable for the 1:2 complexation is expected to be controlled by control of the bulkiness of ligand molecule. Future work will aim to use the unique properties discussed here to develop a Ln separation method.
This study was supported by the New Energy and Industrial Technology Development Organization (NEDO).
<Previous: 5-7 | Next: 6 HTGR Hydrogen and Heat Application Research>