Hydrides of elements in the second- and third-row of the periodic table like CH4, NH3, H2O and H2S are stable molecules formed by a covalent bond with eight valence electrons. However, the situation changes dramatically when the hydrogen atoms in these molecules are replaced with lithium atoms. For instance, replacement of the hydrogen atoms in H2O by lithium atoms gives Li3O with nine valence electrons. This molecule, discovered in 1978 by a JAERI researcher in collaboration with German scientists, is thermodynamically more stable than the octet molecule Li2O with eight valence electrons, and is called a "hyperlithiated" molecule. Following the discovery of Li3O, results of theoretical calculations predicted the existence of such hyperlithiated molecules as CLi6, Li4N, Li4O, Li4P, Li3S, Li4S and Li2F. All but Li4N and Li2F molecules have been detected by Knudsen-effusion mass spectrometry in JAERI and the structures and bonding have been elucidated.
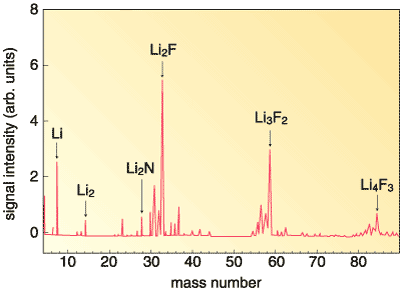
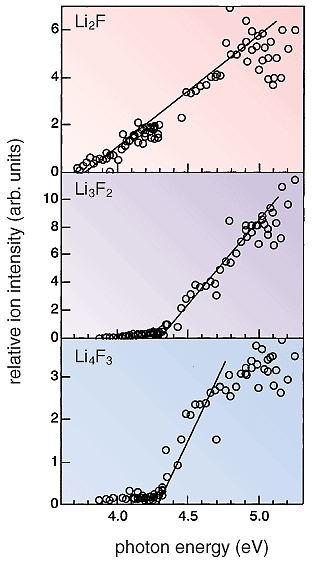
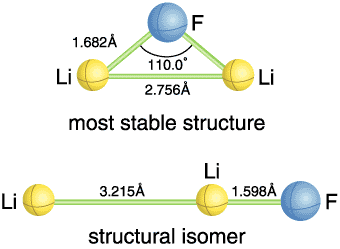
Although Li4N and Li2F have so far hardly been observed, Li2F was eventually detected this year in a supersonic beam of vapors generated by laser ablation of a solid mixture of LiF and Li3N (Fig. 6-1). The ionization energy determined by the experiment (Fig. 6-2) confirms the existence of Li2F with nine valence electrons. In addition, new clusters Li3F2 and Li4F3 with an excess electron were detected in the supersonic beam. Figure 6-3 illustrates the theoretically deduced structure of hyperlithiated Li2F. It is hoped that this study on the structure and stability of Li2F will contribute to further understanding of the nature of chemical bonding. |