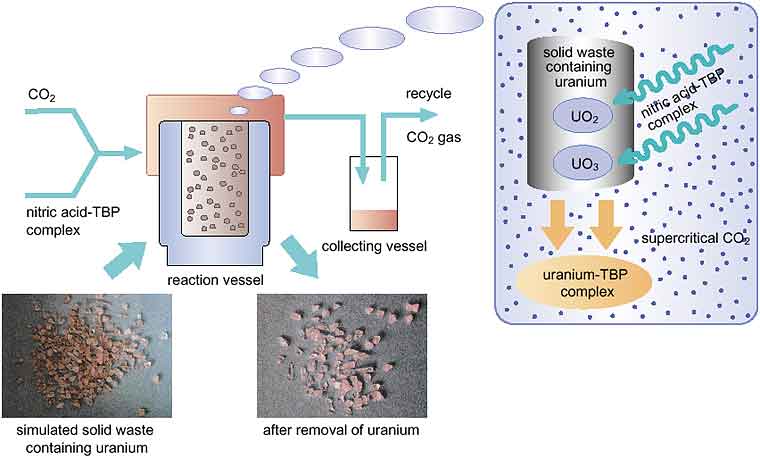
| Fig. 4-16 | Removal of uranium from solid waste by the supercritical carbon dioxide fluid leaching (SFL) method Uranium in solid waste can react with a reagent (a complex of nitric acid and TBP) to form uranium-TBP complexes in a supercritical carbon dioxide phase in a reaction vessel at 50 - 60 degrees cent. and a pressure of 100 - 200 atmospheres. The uranium-TBP complex formed is dissolved into the supercritical carbon dioxide. In the collection vessel at ambient temperature and pressure, the carbon dioxide gasifies and the uranium-TBP complex is recovered. Using this method, we have been able to remove uranium effectively from a simulated solid waste which was a mixture of uranium oxide (100 - 200 mg) and sand (50 g) (photo). |
| Go back by your web browser, or click the right button. |  |
| Persistent Quest - Research Activities 2001 Copyright(c) Japan Atomic Energy Research Institute |