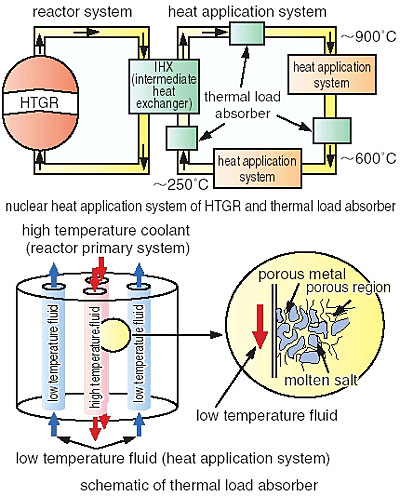
The high temperature gas, 950 degree Celsius, generated by the High Temperature Gas Cooled Reactor (HTGR) is expected to be utilized to produce chemical products. The chemical production system connected to the HTGR is called a nuclear heat application system. To increase the reliability of the system (including the reactor) during operation, transient temperature variations (thermal load variations) generated by irregular operation of the nuclear heat application system should be minimized so as not to effect the operation of machinery including the reactor. A steam generator could be effectively used for low temperature applications, below the critical temperature of water (374 degree Celsius). When two or more nuclear heat application systems are connected in cascade to an HTGR, however, a thermal load reduction of the upper system will likely occur in the higher temperature region, above the critical temperature of water. Thus, a steam generator cannot be used. The concept of using the latent heat of a phase change material (PCM), such as a molten salt, which will absorb heat during the phase change from solid to liquid (Fig. 1-16) has been suggested.
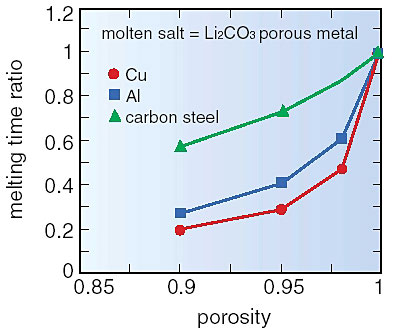
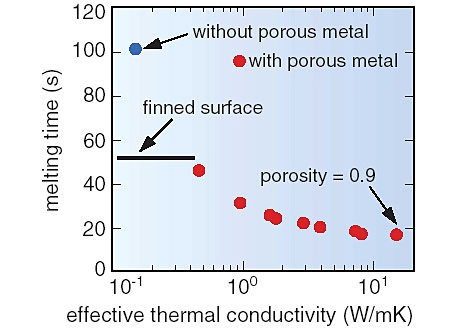
When heat is mainly transferred by conduction in the molten salt, it is necessary to improve heat transfer for fast reduction of thermal load variations because of the low thermal conductivity of molten salt. For this purpose, we propose to improve the effective thermal conductivity of molten salt by immersing high porosity material with a high thermal conductivity, such as metal foam, in the salt. This can increase effective thermal conductivity of the molten salt by a factor of several to one hundred times the normal value. The melting times with the improved thermal conductivities were estimated by numerical analysis. The results are shown in Figs. 1-17 and 1-18 for Li2CO3 as a PCM. Considering the mass reduction of the PCM due to the existence of porous material, the melting time was reduced to one-half to one-fifth for a porosity of 0.9 (Fig. 1-17). In this case, an increase in heat transfer is about three times that when a finned surface is used. When the thermal conductivity of a PCM is very low, an increase in heat transfer could be several decades higher than for a finned surface. This effect is attained because surface temperature of the heat storage medium can be maintained at lower temperature by the immediate transfer of heat by heat transfer from a metallic surface by conduction. Coupling this technique with a finned surface would attain an even greater improvement in heat transfer.
|