In D-T fusion reactors, fast neutrons and helium (He) particles are formed by the nuclear reaction. Fast neutrons produce thermal energy, while He is useless and is classified as 'the ash.' If He accumulates in the core of a reactor, normal operation of the reactor is impeded resulting in a reduction in nuclear power. It is desirable for He to be selectively exhausted from the core of the reactor, but vacuum pumps presently do not have the ability to separate mixed gases. Therefore, it is necessary to develop a technology by which unused fuels (deuterium: D and tritium: T) are separated and collected from the exhaust gas for the reuse as fuels.
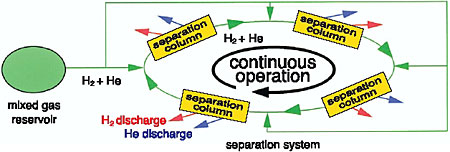
To meet this requirement, we have developed a new method named the Continuous Circulation Chromatograph method (C3 method) (Fig. 2-22). The device consists of several adsorbent columns, as shown in Fig. 2-23, to separate a mixed gas into component gases using the difference in adsorption affinity among the component gases, and open-closed valve timing to isolate separated gases.
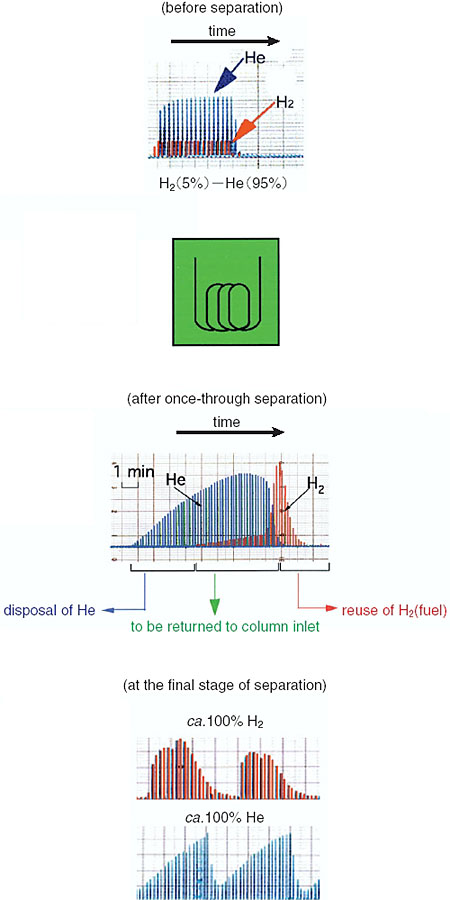
So far, we have demonstrated the separation of various H2-He and D-He mixtures into their component gases using a molecular sieve as the adsorbent (Fig. 2-24). This method will be applied to the separation of T-He mixtures. The impurity levels in the separated gases are lower than the detectable limit of a mass spectrometer. The new method has a safety advantage because the operation is performed below atmospheric pressure.
By applying the method to a fuel cycle system of a fusion reactor, the fuel utilization efficiency will be increased. Further, applying the method to a fuel cycle system will lower the cost of construction due to its simplicity.
Besides fusion applications, we have confirmed that the C3 method can be used for the separation of perfluorocarbon compounds, which is one of the important issues when addressing the earth-warming problem, by changing the molecular sieve to an activated charcoal as the adsorbent. This method is now being refined for practical applications.
|