|
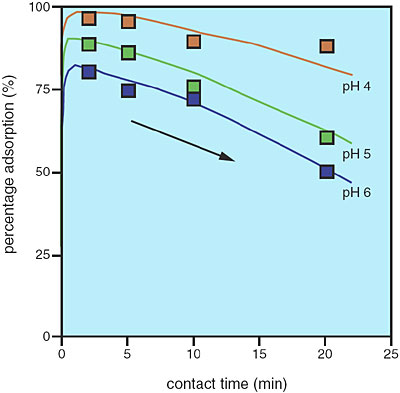
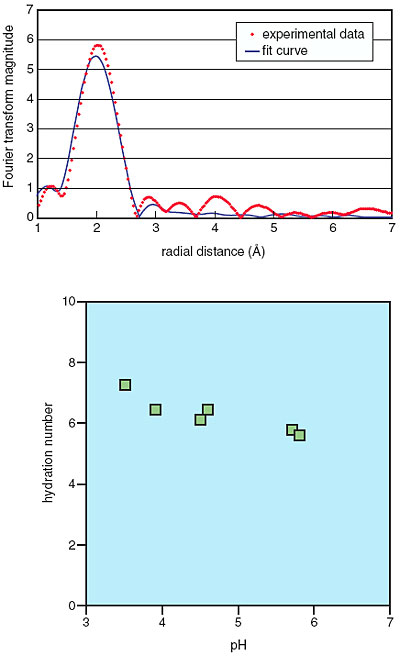
The cell surfaces of microorganisms have affinities for various metal ions. Adsorption of metal ions on cell surfaces with this affinity facilitates their subsequent absorption into the cell. The cell surface attracts not only ions important for the life support of microorganisms, but also unwanted ions. The fate of the adsorbed metal ions is of great interest. We have investigated the association of a unicellular green alga, Chlorella vulgaris, with an unwanted ion, Eu(III). Our kinetics study has shown that maximum adsorption of Eu(III) on C. vulgaris was attained within three minutes of contact; afterward, the percentage adsorption decreased with time (Fig. 7-14). Extended X-ray absorption fine structure (EXAFS) studies and time-resolved laser-induced fluorescence spectroscopy (TRLFS) studies showed that the short-term adsorption of Eu(III) on C. vulgaris was attributable to its coordination on the algal cell wall and not on the cell membrane (Fig. 7-15). Analysis of the alga-metal mixture solution after contact showed that C. vulgaris exudes some organic substances with a high affinity for Eu(III). These findings indicate the existence of a "catch-and-release" detoxification mechanism, which functions in response to the adsorption of unwanted ions on the cell. Thus, any unwanted ions caught are later released by being coordinated with the exudates. It is expected that the exudates have a high selectivity for metal ions, because they are produced and released after the cells have recognized the metal ions adsorbed. Utilization of such exudates could lead to development of a novel metal recovery system, which is both ecologically friendly and cost effective.
|