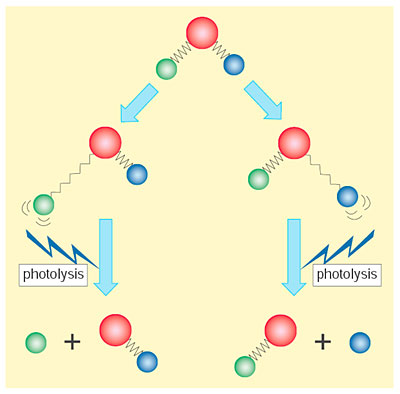
If a specific atom can be eliminated from a molecule selectively, it becomes possible to create materials with new functions. Selective elimination of a target atom may be realized by selective excitation of a stretching vibrational mode, in which the chemical bond which includes the target atom is expanding and contracting. Photolysis of the stretching vibrational state can lead to preferential elimination of the target atom as a result of the stretching motion (Fig. 8-1).
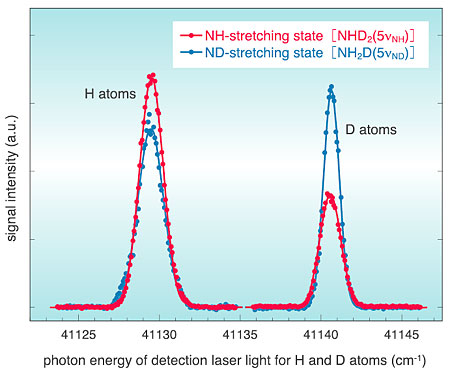
For ammonia molecules containing both H and D atoms (NH2D or NHD2), we have investigated the ultraviolet photodissociation at a wavelength of 243.1 nm which follows the excitation into a stretching vibrational state induced by laser irradiation. By comparing the yield of the eliminated H atom with that of the D atom, the branching ratio between the NH- and ND-bond dissociation channels has been determined. It was found that the branching ratio is substantially different between the NH-stretching (5nuNH) and ND-stretching (5nuND) vibrational states, as shown in Fig. 8-2. The NH-stretching excitation leads to preferential breaking of the NH bond (red line), whereas the ND-stretching excitation leads to preferential ND-bond breaking (blue line). These results indicate that the target H or D atom can be preferentially eliminated from the molecule, through use of the photolysis of the vibrationally excited state in the stretching mode. It is expected that this method will be useful for the production of isotopically controlled materials.
|