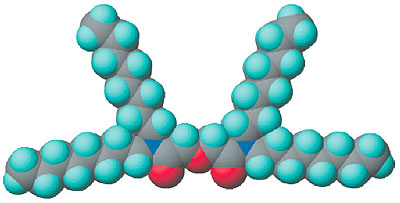
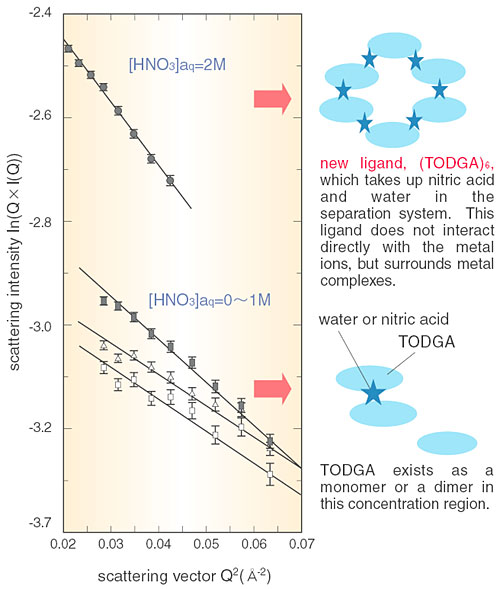
Americium (Am) and curium (Cm) are long half-life, alpha-emitter nuclides, possibly injurious if they are absorbed into the human body. Thus, these nuclides need to be removed from high-level radioactive waste and to be transmuted into short half-life nuclides. For this purpose, N,N,N',N',-tetra-n-octyl-diglycolamide (TODGA), a separation reagent for these elements, has been developed by JAERI. It is well known that a "multidentate extractant," having plural biding sites such as oxygen, is effective for separation of trivalent lanthanides and actinides. The tridentate extractant TODGA, having three oxygens as binding sites (Fig. 8-10), has been reported to be highly superior to bidentate extractants for separation of these elements. Why TODGA shows such good efficiency with the increase of a single binding site was a challenge to our understanding. The size and the number of molecules in the extracted complex have been determined by small angle neutron scattering (SANS). From the slope and intersection of such data, as shown in Fig. 8-12, the form of the extracted complexes has been determined on the molecular level. Surprisingly, we have found that the TODGA had grown into "another new extractant" by aggregation of TODGA extractants and extracted complexes.
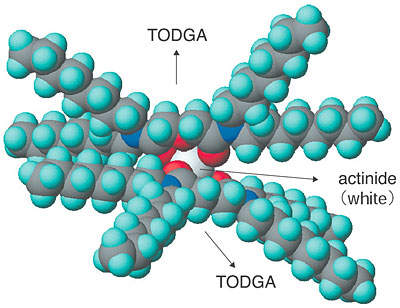
In the separation of actinides by extraction with TODGA, two TODGA complexes interact with each actinide, whereupon the actinide can dissolve into an organic phase as [An(TODGA>)2(H2O)3](NO3)3 (Fig. 8-11). As a result, the actinides can be separated from the aqueous phase. In the earliest stages, TODGA is just a normal extractant. However, TODGA then forms a new species of extractant (TODGA)6 by utilizing water and nitric acid as a bonding agent in the concentration range of nitric acid more than 2M. This "new extractant" greatly enhances the actinide extraction efficiency. The extractability of actinides using TODGA has become the best available in the world today.
|
 ): 0M, (
): 0M, ( ): 0.1M, (
): 0.1M, ( ): 1M, (
): 1M, ( ): 2M.
): 2M.