First of all, let us call to mind the process of water electrolysis which generates hydrogen and oxygen. In this reaction, the input of electrical energy leads to decomposition of water into hydrogen and oxygen. Conversely, these two gases, when they take part in a direct electrochemical reaction, produce electric power as well as water. Such an elegant device is called a fuel cell.
Fuel cells are classified into 4 or 5 types according to their ion-conducting electrolyte materials and operating temperature. Among these a polymer electrolyte membrane fuel cell (PEMFC) has attracted considerable attention because of its various advantages such as low operating temperature, high energy efficiency, and high power density. The PEMFCs have, as their electrolyte, an ion-exchangeable polymer membrane, and so their development has been targeting wide applications in the transportation, stationary, and portable power fields. The ion-conducting electrolyte membrane is a key component in the PEMFCs; this acts as a separator to prevent mixing of the reactants between the electrodes. Therefore, high ion conductivity and stability under exposure to the fuel are of great importance for improving the fuel cell performance.
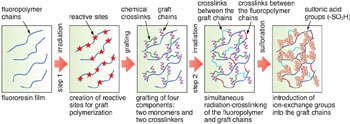
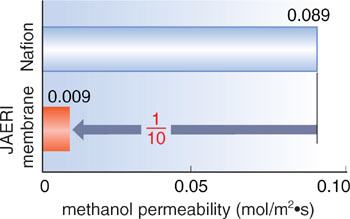
At the beginning, styrene-grafted, sulfonated membranes were developed for fuel cells to which the most promising hydrogen source, i.e., methanol, is directly supplied. But these showed low durability and lacked controllability of the methanol permeation. To solve this problem, therefore, we have recently established the radiation-graft copolymerization of two monomers as an alternative to styrene, offering high chemical stability and ion conductivity, and two crosslinkers for dense network structures and flexibility. In addition, such four-component grafted films were irradiated with gamma-rays at room temperature to crosslink the fluoropolymer and graft chains at the same time (Fig. 6-6). The resulting membranes were found to exhibit twice the proton conductivity and 1/10 the methanol permeability, as compared to commercially-available membranes (Fig. 6-7). In our accelerated test, the membrane durability was six times as high as that of the styrene-grafted ones (achieving a 4200-hour fuel cell operation in a previous report).
Based on the above successful results, our collaborator, Nitto Denko Corporation, will soon be producing large-scale membranes for commercial applications. |