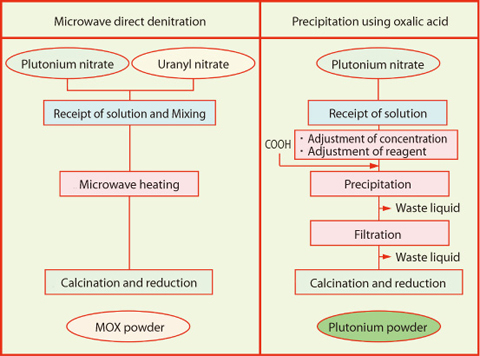
Fig.8-2 Comparison of conversion methods
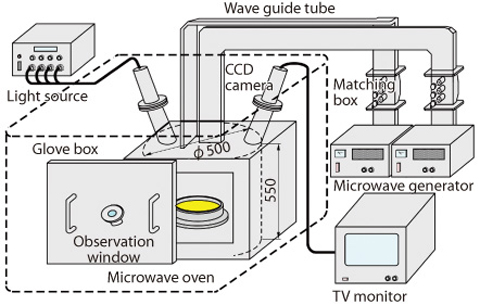
Fig.8-3 Schematic view of denitration testing apparatus
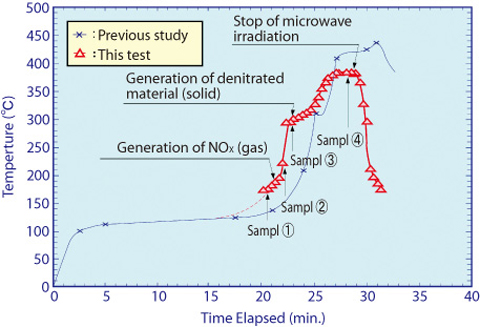
Fig.8-4 Temperature of uranium compounds during microwave irradiation
Spent fuel from nuclear power reactor is chemically treated in reprocessing plants, and then plutonium and uranium are recovered as reusable sources. In Europe, recovered plutonium is converted to the oxide powder by precipitation using oxalic acid and mixed with uranium oxide powder, followed by the mixed oxide (MOX) fuel fabrication. A new co-conversion method where plutonium and uranium are converted directly to MOX powder after mixing them in solution state was developed and adopted in Japan (Fig.8-2). This process has the feature of nuclear weapons proliferation resistance because there is no pure plutonium oxide powder in the process. This feature is exactly consistent with the policy of Japan that nuclear power should be used only for peaceful purposes. The microwave denitration method excites molecules by microwaves just like a household microwave oven, and nitrate is decomposed more rapidly by internal heating than by external heating. As a result, the produced powder is made up of fine particles which are suitable for sintering into high density pellets.
When mixed plutonium nitrate and uranium nitrate solution is heated, water and nitric acid evaporates at the first stage, then crystallization water of nitrates and nitrate itself are decomposed, and MOX remains at the end. In this study, we developed a special device to measure non-invasively and selectively the temperature of uranium material regardless of the moisture or NOx gas in the oven (Fig.8-3). The purpose of the development was that the conventional infrared thermometer could not measure temperature correctly because infrared rays were absorbed by moisture or NOx gas between the measured substance and the thermometer. The device was attached to the oven and temperature transition of the material was accurately measured. Then, samples were taken from each stage of reactions and thermometric analysis and X-ray analysis were carried out (Fig.8-4). As a result, we could confirm the chemical reactions formulae shown below :
UO2(NO3)2·6H2O→UO2(NO3)2·3H2O+3H2O
UO2(NO3)2·3H2O→UO2(OH)NO3+HNO3+H2O
UO2(OH)NO3→β-UO3+0.5H2O+NO2+0.25O2
And/or UO2(OH)NO3→β-UO3+HNO3