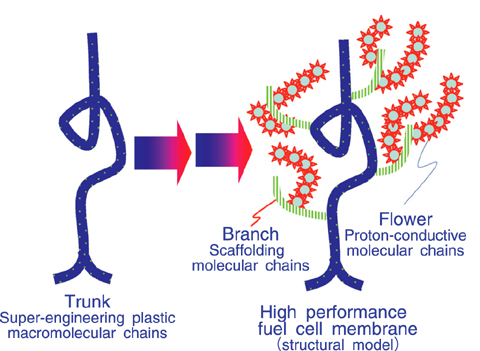
Fig.4-21 Schematic diagram for the development of high- performance fuel cell membranes by radiation grafting method
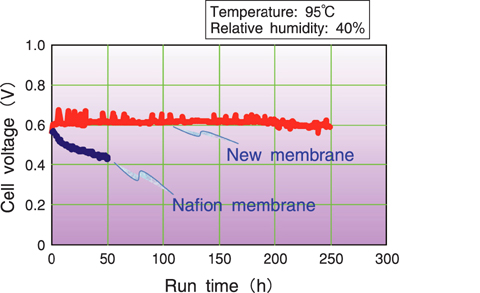
Fig.4-22 Fuel cell performance of new polymer electrolyte membranes operated under high temperature and low humidification
The polymer electrolyte membrane fuel cells are promising electric power sources for home cogeneration and automobiles. To enhance the fuel cell performance and catalyst utilization efficiency, and to simplify the cooling system, it is desired that the fuel cell be operable at high temperature and low humification. However, although the commercial perfluorinated polymer electrolyte membranes, such as Nafion membranes, have excellent performance below 80°C and with full humidification, performance deteriorates remarkably above 90°C or at relative humidity below 50%. Therefore, development of a new polymer electrolyte membrane that can be used for thousands of hours under high temperatures and low humidification has been widely pursued recently.
To meet the demand for high temperature fuel cells, we used a thermally stable engineering aromatic polymer as the base film to develop a polymer electrolyte fuel cell membrane. In this study, the typical super-engineering film polyetheretherketone (PEEK) was chosen as the base film, and a styrenesulfonic ethyl ester (ETSS) monomer was radiation-grafted to this. The ETSS-grafted PEEK film was hydrolyzed to obtain the desired high performance polymer electrolyte membrane. By means of this new method, the PEEK-based polymer electrolyte membrane was directly prepared without a severe sulfonation reaction using a strong acid. Consequently, the intrinsic properties of PEEK were maintained and the resulting polymer electrolyte membrane was very tough, suited for application in fuel cells.
However, because of the low grafting activity of the PEEK chains (trunks), the ETSS monomer (flowers) was difficult to attach to the PEEK trunks in the desired amounts. Therefore, we designed a branch which could be easily attached to the trunks and easily graft with ETSS flowers. We found that the divinylbenzene (DVB) can serve as this branch well, allowing many flowers to be radiation grafted to the PEEK trunks. As a result, new polymer electrolyte membranes with high proton conductivity and highly thermal durability were successfully developed (Fig.4-21).
The new polymer electrolyte membrane was tested in a fuel cell under high temperature and low humidity (cell temperature, 95°C; 40% relative humidity). For comparison, the Nafion membrane was also tested under the same conditions. It was confirmed that the new membranes exhibited higher cell performance than the Nafion membrane. The membrane durability was evaluated in a fuel cell operated at a fixed current density of 0.3 A/cm2. The Nafion membrane cannot withstand such a high temperature and low humidity, its voltage drastically dropping in a short operation time due to the deterioration-induced crossover of the fuel across the membrane. In contrast, the developed new polymer electrolyte membranes had significantly higher durability and stability. The voltage of the fuel cell was almost unchanged after the initial 250 h of operation, and was estimated to be stable for more than thousands of hours under this high temperature and low relative humidity (Fig.4-22).
In conclusion, it was possible to develop a polymer electrolyte membrane for a fuel cell which has high performance under high temperature and low humidification, by radiation grafting techniques. Development of such a highly stable and durable polymer electrolyte membrane is a big breakthrough for the practical application of fuel cells using hydrogen as the fuel.