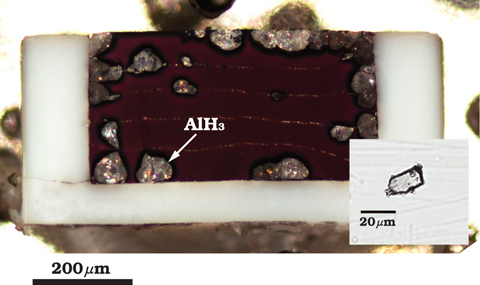
Fig.4-6 Optical micrograph of sample after treatment
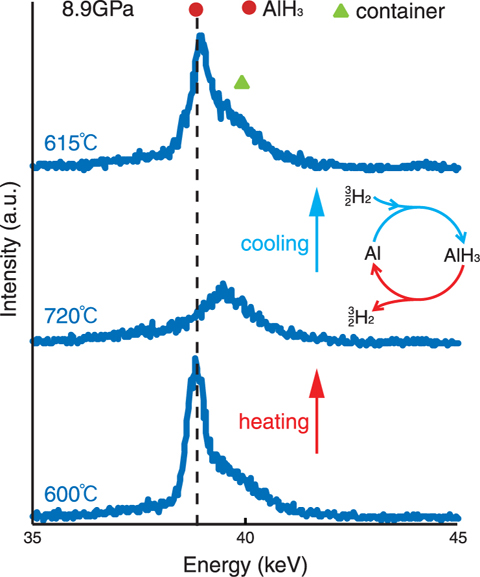
Fig.4-7 Powder X-ray diffraction profiles of aluminum sample immersed in high-pressure high-temperature hydrogen fluid
Hydrogen is an ideal energy carrier since it minimizes harmful effects on the environment. Development of a safe and efficient storage system of hydrogen is widely recognized as a key technological challenge which must be met to realize a hydrogen-based energy economy. Hydrogen can be stored as a pressurized gas, cryogenic liquid, and solid fuel; for example, hydrogen forms metal hydrides with some metals and alloys. These solid-state storage media provide a safety advantage over the gas and liquid storage methods.
Typical hydrogen storage alloys, such as LaNi5, absorb hydrogen at relatively low pressure. In contrast to such hydrides, high pressure is needed to hydrogenate aluminum. Passivation film on the surface of aluminum also prevents the hydrogenation reaction. AlH3 is, however, promising as a hydrogen storage material due to its large hydrogen content (10.1wt%). If the hydrogenation of aluminum is realized, it is expected to develop novel aluminum-rich alloy hydrides with moderate hydrogenation pressure.
We have succeeded in hydrogenation of aluminum and recovery of AlH3 (Fig.4-6). The hydrogenation and dehydrogenation process was investigated by in situ X-ray diffraction measurement at SPring-8 (Fig.4-7). Highly reactive hydrogen fluid suppressed the influence of the chemically stable oxide layer on the aluminum surface.
In the future, we will elucidate the hydrogenation and dehydrogenation mechanism of aluminum, and develop novel aluminum-based alloy hydrides which can absorb hydrogen at practical pressure temperature conditions.
The present study was conducted as part of the "Advanced Fundamental Research Project on Hydrogen Storage Materials" commissioned by the New Energy and Industrial Technology Development Organization (NEDO).