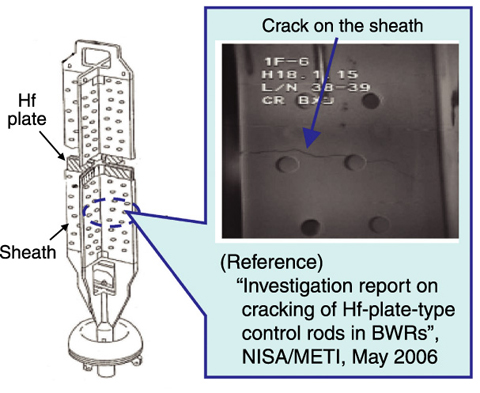
Fig.6-6 Structure of hafnium (Hf)-plate-type control rods and example of sheath cracking
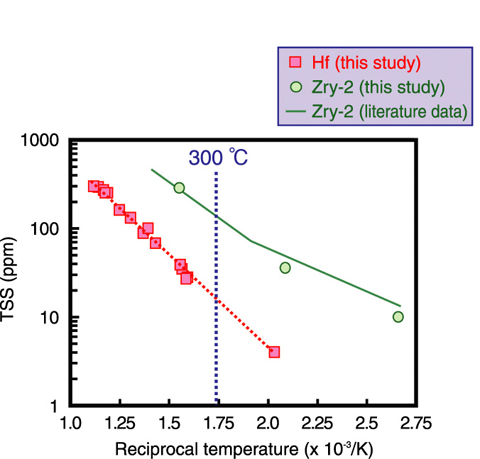
Fig.6-7 Temperature dependence of terminal solid solubility of hydrogen (TSS): a comparison
between Hf and zircaloy-2 (Zry-2)
Control rods in a reactor core are exposed to a severe environment, such as high pressure water at high temperature and high radiation, for a long time. Control rods made of boron, which is a neutron absorber, usually have to be exchanged frequently, leading to the production of high-level radioactive waste. Because hafnium (Hf) has a considerably longer nuclear lifetime than boron and is resistant to corrosion, it has been used as a neutron absorber for long-life control rods.
Recently, cracking which may have resulted from irradiation-assisted stress corrosion cracking was found in the stainless steel cladding (sheath), etc., of Hf-plate-type control rods (Fig.6-6). Size change of the Hf plate due to irradiation growth and hydride precipitation is considered to be a possible cause of the cracking. Countermeasures such as replacement of the control rod, etc., were performed. In order to use Hf control rods safely for a long time, we investigated the quantitative evaluation of Hf size stability.
Hf absorbs hydrogen through a corrosion reaction, and then part of the absorbed hydrogen precipitates as hydrides. Because the hydride precipitation causes size change (volume expansion), it is important to know both the temperature and hydrogen concentration conditions at which hydrides precipitate in Hf. Fig.6-7 shows the terminal solid solubility of hydrogen (TSS) in Hf, i.e., the lower limit of the hydrogen concentration at which the hydrides precipitate. Results for zircaloy-2 (Zry-2), which has similar chemical properties and is used for fuel claddings, is also shown in this figure for a comparison. The TSS for Hf shows similar temperature dependence to that for Zry-2. The TSS for Hf at 300 °C, which corresponds to the operational temperature of control rods, is about 20 ppm. The hydrides were found to precipitate in Hf under a lower hydrogen concentration condition than Zry-2. We will clarify the effect of hydride precipitation on the size change of Hf.
This research is a part of a program of the Nuclear and Industrial Safety Agency (NISA) of the Ministry of Economy, Trade and Industry (METI).