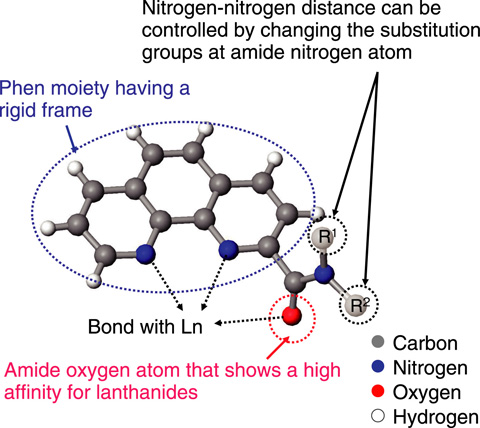
Fig.4-15 New lanthanide-recognition compound PTA
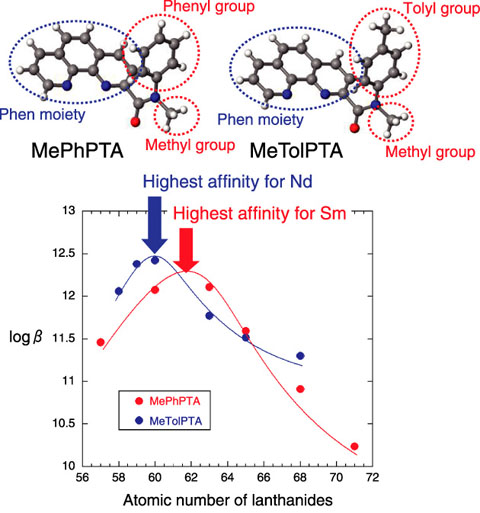
Fig.4-16 PTA consisting of different substitution groups and its affinity for lanthanides
Lanthanides (Ln) are essential for high-technology industries, because they contribute to the performance improvement and/or size and weight reduction of high-technology products. This is possible because of the unique chemical properties of Ln. To further improve the performance and/or save energy of such products, it is important to improve the purification technique for Ln materials. However, all Ln elements have same stable valence states and similar ionic radii: therefore, the intra-series separation of Ln is extremely difficult. In this study, we synthesized a novel organic ligand PTA (= N-alkyl-N-phenyl-1,10-phenanthroline-2-carboxamide, Fig.4-15) which exhibits strong bonding with Ln, and by utilizing PTA, we succeeded in recognizing a slight difference in the ion size of Ln.
PTA is mainly composed of an amide group and a phenanthroline (Phen) moiety. An amide oxygen atom shows a high affinity for Ln, and an amide nitrogen can be substituted by various substitution groups. The Phen moiety has a rigid frame that is not easily expanded, contracted, or twisted, and can bond with Ln through two nitrogen atoms. Generally, the affinity of Phen for metal ions decreases significantly in acidic conditions because of the protonation of nitrogen atoms. However, in the case of PTA, the nitrogen atoms in the Phen moiety show a high affinity for Ln even in highly acidic conditions because of the effect of the amide oxygen atom that is not easily protonated and strongly attracts Ln even in acidic condition[1]. Furthermore, it has been revealed that the selectivity tendency of PTA for Ln changes upon modifying the substitution groups at the amide nitrogen. For example, PTA consisting of methyl and phenyl groups as substitution groups at the amide nitrogen shows the highest affinity for Sm: in contrast, a PTA in which the phenyl group is replaced by a tolyl group shows the highest affinity for Nd (Fig.4-16)[2]. In other words, the suitable ion size for complexation changes upon changing the substitution groups. In PTA, the change in substitution groups causes a slight change in the nitrogen-nitrogen distance in the Phen moiety. Thus, we synthesized some PTA derivatives having different substitution groups and investigated the chemical structures of these Ln complexes by using X-ray crystallography and other methods. The results revealed that a correspondence between the ion size of Ln and the form of the rigid Phen moiety is required for strong bonding between them. Moreover, the suitable ion size can be controlled by the modulation of the form of the Phen moiety (nitrogen-nitrogen distance), followed by the modification of substitution groups.
The separation of Ln on the basis of the ion-size recognition property of PTA will facilitate the establishment of an innovative single-element separation technique for Ln.