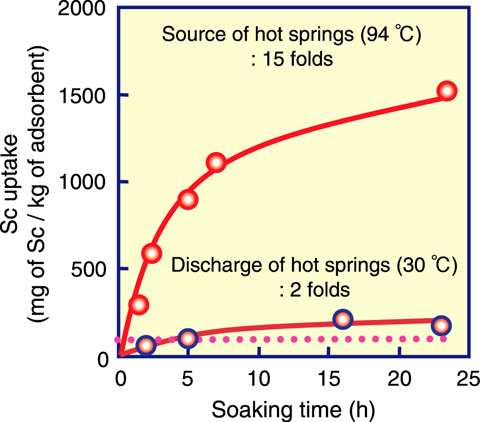
Fig.4-17 Amount of scandium (Sc) uptake as function of contact time in hot springs
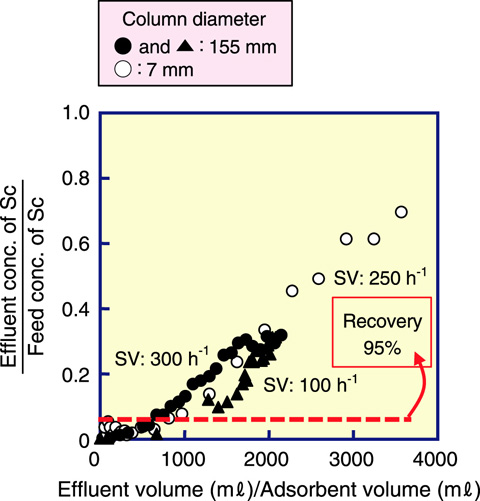
Fig.4-18 Breakthrough curves showing effects of flow rate on lab scale (7 mmφ) and on pilot scale (155 mmφ)
Rare-earth elements are defined as the 17 elements comprising scandium (Sc), yttrium, and 15 lanthanides. In the earth's crust, Sc is the 30th most abundant element. Sc is called a rare-earth metal not because it is particularly scarce but rather because it is difficult to obtain. Ores containing Sc as the main component are extremely rare, and Sc is primarily obtained as a by-product of uranium extraction from uranium ores. The conventional processes for recovering useful rare-earth metals have the disadvantages of not only environmental pollution but also poor recovery efficiency; therefore, finding adsorbents that can efficiently recover these metals, particularly Sc, by adsorption is necessary. This is so because during the recovery of a substantial amount of metal ions, no slag is produced during the collection process because the adsorbent collects the metal directly from solution.
Hot springs are an integral part of Japanese culture. Approximately 3000 hot springs are located in Japan, which have long been appreciated and enjoyed by the people of Japan. In addition, hot springs contain many dissolved metals such as vanadium and Sc. If these metals could be collected from hot springs, the industrial demand for these metals would be covered. A suitable metal adsorbent can be synthesized by a radiation-induced graft polymerization technique on a fibrous trunk. Fig.4-17 shows Sc uptake as a function of contact time. After contacting in a hot springs for 23 h, the Sc uptake reached 220 mg of Sc/kg of adsorbent from the hot spring discharge, and 1500 mg of Sc/kg of adsorbent from the hot spring source. These values correspond to 2 times and 15 times the Sc content of 100 mg/L in an ore, respectively. On the basis of the data obtained from batch adsorption, we set up pilot-scale (115 mmφ) adsorption equipment at a hot spring site in Kusatsu Town (Gunma Prefecture, Japan). In the column mode test, the column was placed such that the hot spring water passed through the column with flow rates of 100 and 300 h-1 SV (space velocity). The breakthrough curves are essentially independent of the flow rate, as shown in Fig.4-18. The breakthrough point and capacity were almost same as those on a lab scale (115 mmφ).
When we selected a fibrous material as a trunk polymer, the adsorption rate was extremely higher than that for a commercial resin. This implied that the space required for water treatment equipment could be reduced. The advantage of a minimized space is that it makes it possible to install such equipment into a narrow space such as that in a hot springs resort.