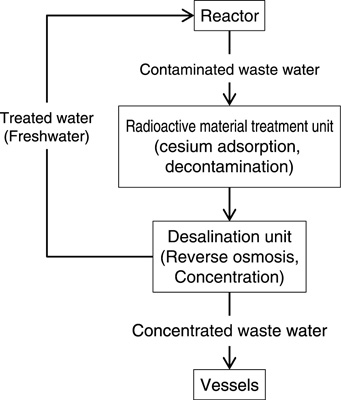
Fig.1-45 Treatment of contaminated water at the 1F
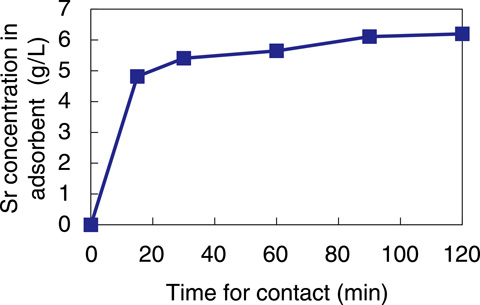
Fig.1-46 Increase in the Sr concentration in the adsorbent
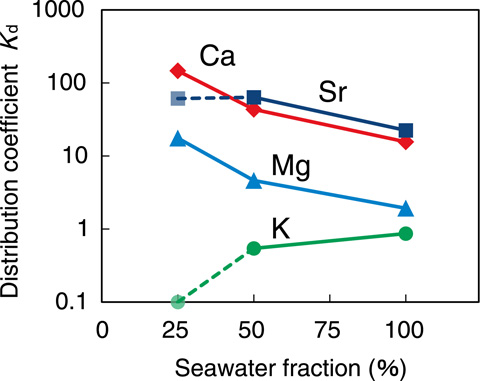
Fig.1-47 Distribution coefficients (Kd) for some elements
At the TEPCO’s Fukushima Daiichi NPS (1F), an enormous volume of contaminated water has been generated and temporarily stored at the plant site. As shown in Fig.1-45, contaminated water bearing radioactive nuclides such as 137Cs is treated by adsorbing Cs into a zeolite using Cs adsorption units, followed by purification using a reverse osmosis membrane. The water is then used for cooling the fuel inside the reactors. The resulting waste water is stored in newly fabricated vessels. The amount of waste water will increase with consecutive treatments; therefore, treated fresh water will be released to the environment after confirmation that the concentrations of radioactive substances are sufficiently low. Among the radionuclides in the waste water, 90Sr is important because it has a low concentration limit and requires an effective decontamination technology.
We developed a treatment process for the low-level liquid waste from the Tokai Reprocessing Plant that utilizes a titanium oxide adsorbent (READ-Sr) for 90Sr decontamination. The contaminated water at the Fukushima site contains seawater, and thus, Sr must be adsorbed in the presence of various metallic cations. Because the READ-Sr adsorbent was developed for a sodium nitrate solution, its application to the waste water with seawater was investigated.
The properties of READ-Sr for adsorption were examined using simulated solutions. As shown in Fig.1-46, for Sr adsorption from water containing 50% seawater, the Sr concentration in the adsorbent reached equilibrium within 30 min. Thus, it was verified that a practical adsorption rate was obtained for Sr adsorption. Sr is an alkaline earth element, and seawater contains chemicals resembling Ca and Mg; therefore, to efficiently remove Sr, the adsorbent must be selective for the element. As shown in Fig.1-47, the distribution coefficient (Kd) is affected by the fraction of seawater, although Sr adsorption and selectivity for Sr and Ca over Mg and K were observed.
The adsorbent was also examined for batch-wise adsorption combined with the Cs adsorbent and in a column test. The Sr decontamination expected based on the distribution coefficients was observed in both tests.
We will continue this study with the aim of applying this technology at the 1F.