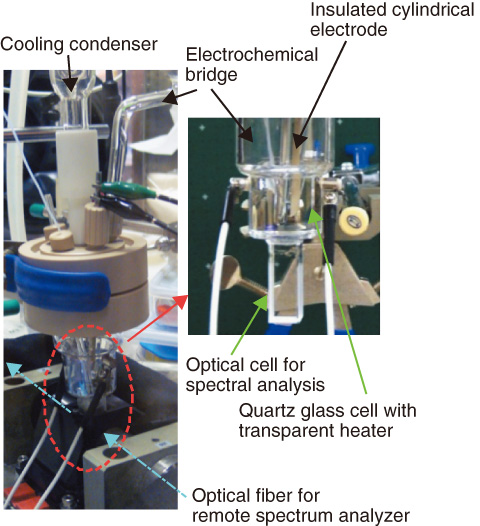
Fig.8-7 Appearance of developed electrochemical cell including optical cell for spectral analysis
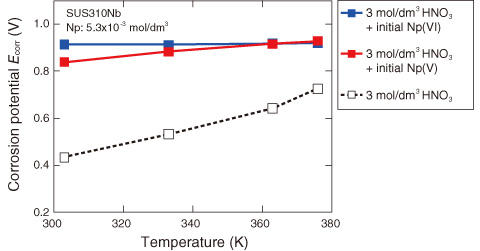
Fig.8-8 Effect of temperature on corrosion potential of stainless steel (SUS310Nb)
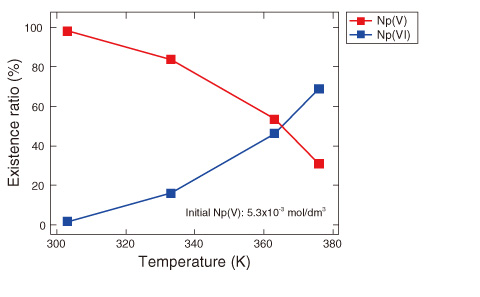
Fig.8-9 Effect of temperature on valence change in Np
Nuclear reprocessing has been promoted for the reuse of uranium (U) and plutonium (Pu) in spent reactor fuels worldwide. A corrosive nitric acid solution is used in nuclear reprocessing, which involves materials containing metallic ions that accelerate corrosion. Therefore, accelerated corrosion of stainless steel, one of the component materials, is an issue in nuclear reprocessing plants.
It is well known that among metallic ions, Pu and neptunium (Np), which are oxidized in boiling nitric acid solution to metallic ions in higher oxidation states, accelerate corrosion the most. Furthermore, the amount of Pu and Np that can be handled in an experiment is restricted because they are radioactive elements.
We developed a specially designed small electrochemical test cell integrated with an optical cell for spectroscopic analysis in order to concomitantly evaluate the oxidation states of Np ions and the polarization curves of stainless steel in boiling nitric acid solution. The goal of this study is to understand the corrosion acceleration mechanism of stainless steel.
The test cell shown in Fig.8-7 enables (1) the handling of a small amount (ca.15 cm3) of test solution, (2) simultaneous spectroscopic characterization and electrochemical monitoring of the solution, and (3) stable electrochemical measurements in a boiling nitric acid solution.
Fig.8-8 shows that the corrosion potential Ecorr of the stainless steel increases with increasing temperature when the solution contains Np ions. The presence of Np ions accelerates the corrosion of stainless steel by increasing the corrosion potential. Further, the corrosion potential in the solution containing Np(VI), in a higher valence state, does not change with increasing temperature, whereas that of a solution containing Np(V), in a lower valence state, increases.
Fig.8-9 shows the existence ratios of Np(VI) and Np(V) in 3 mol/dm3 HNO3 with initial Np(V) ions at various temperatures. The existence ratio of Np(VI) increases with increasing temperature.
In conclusion, Np(VI) oxidized by nitric acid was found to raise the corrosion potential and accelerate the corrosion of stainless steel.
This work was part of work commissioned by the Japan Nuclear Energy Safety Organization (JNES).