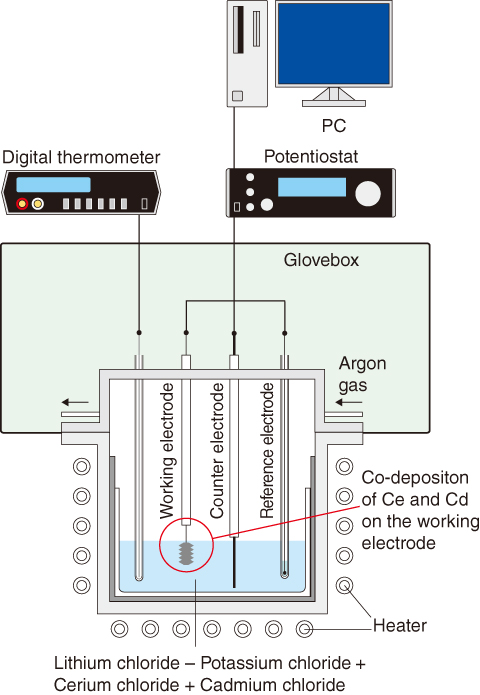
Fig.4-11 Concept of the electrochemical method applied in the present study
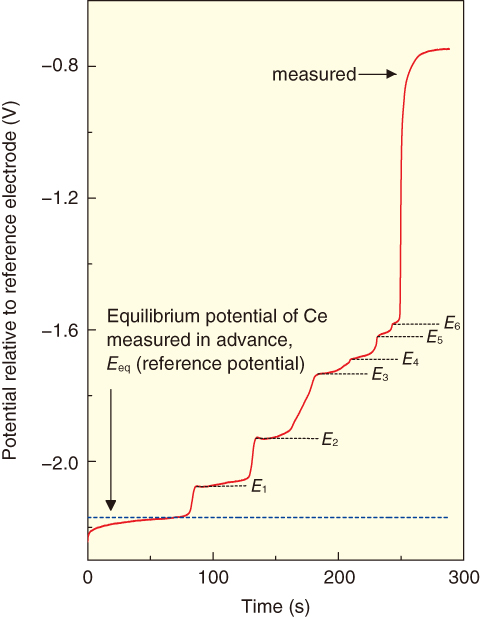
Fig.4-12 Chronopotentiogram of equilibrium potential of Ce-Cd intermetallic compounds relative to reference electrode (673 K)
Partitioning and transmutation is a promising concept for advanced nuclear fuel cycles. In this approach, minor actinides (MAs) are recycled and transmuted to reduce the environmental impact of nuclear waste. Since MAs are highly radioactive and give off significant decay heat, a pyrochemical method is a proper candidate for reprocessing. In the pyrochemical reprocessing method proposed by JAEA, MAs are selectively collected in a liquid cadmium (Cd) cathode in the molten salt bath, following which they are recovered as alloys by Cd distillation and separation. Developing such a pyrochemical reprocessing requires a database of various fundamental properties. Cd distillation and separation requires thermodynamic data on various Cd intermetallic compounds because the decomposition of the intermetallic compounds may be dominant in the last stage of Cd distillation.
Fig.4-11 presents the electrochemical method used in the present study, in which the three-electrode technique was adopted for electrochemical measurements, from which the Gibbs free energy of formation of various intermetallic compounds was determined.
Cerium-Cadmium (Ce-Cd) intermetallic compounds form by co-deposition of Ce and Cd metals on the working electrode. The Gibbs free energy of formation of Ce-Cd intermetallic compounds was determined from the difference between two potentials: the redox reaction potential for Ce3+/Ce and the plateau potential for the Ce-Cd intermetallic compound (E1−E6) in the molten salt (Fig.4-12).
These results represent the first determination of the Gibbs free energy of formation of these six Ce-Cd intermetallic compounds.
Based on the similarity between lanthanide (Ln) and actinide (An), we plan to use An with this electrochemical method and evaluate the Gibbs free energy of formation of An-Cd intermetallic compounds.