Fig.1-10 Comparison of operations at sampling sites
![Fig.1-11 Relationship between EC value of supernatant and [cation exchange resin weight] / [water volume] ratio obtained by the batch method](img/honbun/e2015_1-11.jpg)
Fig.1-11 Relationship between EC value of supernatant and [cation exchange resin weight] / [water volume] ratio obtained by the batch method
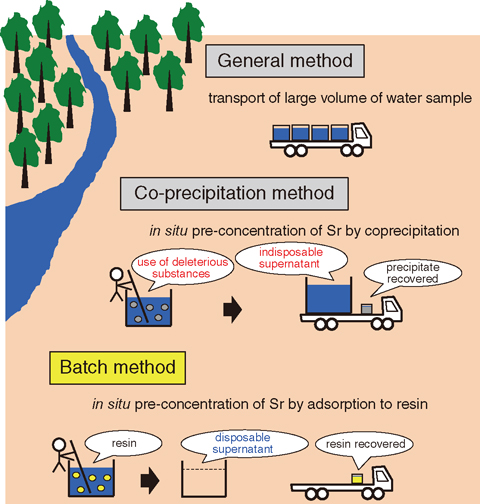
A large volume (over 100 ℓ) is required for precise determination of the low-level radiostrontium activities of freshwater samples. The difficulty of transporting such samples to the laboratory limits the number that can be analyzed. A co-precipitation method using deleterious substances is also undesirable because of the disposal of the waste liquid in the field. In this study, an improved analytical method for low-level radiostrontium in a freshwater sample, which enables sample-collection from many locations, was developed. Emphasis was placed on the in situ pre-concentration of Sr by a batch method using Powdex resin to efficiently reduce the sample volume without using deleterious substances in the field (Fig.1-10).
The batch method used here refers to the operation used to adsorb specific components to resin added into the container of a large water sample under stirring. The challenge of this study was to estimate the amount of resin required for quantitatively adsorbing Sr from water samples with various dissolved solid contents. The results of batch experiments using various environmental water samples indicated that the electric conductivity (EC) of supernatant could be determined by the EC of the original water sample, the amount of cation exchange resin, and the water volume. Because the EC of supernatant was assumed to be zero under complete adsorption of Sr, the amount of cation exchange resin required could be empirically determined by the EC of the original water and the water volume (Fig.1-11). Keeping the pH of supernatant neutral by the simultaneous addition of the anion exchange resin enabled both efficient in situ reduction of sample volume and sampling from multiple locations.
The resin adsorbing Sr was brought back to the laboratory. The Sr was then chemically and radiochemically separated from Ca and interference nuclides of radiostrontium (i.e., radioactive Pb and Ra isotopes) for β-ray measurement after extraction from the resin.
Analysis of tap water samples by this method gave high chemical recoveries (88% on average) of Sr. Some water samples (170 ℓ) incorporating known amounts of 90Sr with different salinities were prepared for validation assessment. The 90Sr activities determined by this analytical method were in good agreement with those added, and validation was confirmed. The detection limit of 90Sr activity with 170 ℓ water samples was estimated to be approximately 0.1 mBq ℓ-1.
The analytical method developed in this study enabled the precise evaluation of internal exposure, and can contribute to ensuring the safety and security of residents.