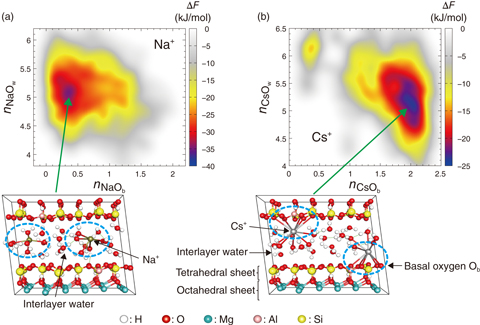
Fig.1-34 The free-energy surface, ΔF, representing the adsorption states of (a) Na+ and (b) Cs+ in the interlayer of 2:1-type tri-octahedral clay minerals obtained from first principles metadynamics and the adsorption structure corresponding to its minimum
The accident at the TEPCO’s Fukushima Daiichi NPS in 2011 released a large amount of radioactive cesium (Cs) into the environment. The resultant Cs deposited onto the ground remains at its subsurface and continues to emit radioactive rays. Large-scale decontamination has been performed at Fukushima, thus reducing the radiation significantly. Thus, an emerging issue that is recognized today is as to how to treat the huge amount of accumulated waste soil.
Cs in the soil is observed to be predominantly adsorbed by weathered biotites (WBs). Hence, the waste volume can be reduced if Cs desorption is realized from them. However, an effective method for volume reduction has not been successfully developed owing to missing fundamental knowledge of Cs adsorption at an atomic scale. It is therefore necessary to elucidate the adsorption states of Cs in WBs for this purpose.
The weathering of mica clay minerals including biotite leads to the loss of some quantity of interlayer cations, which are eventually replaced with water molecules. Therefore, interlayer water is expected to play an important role in the adsorption and subsequent fixation of Cs in the WBs of Fukushima. We systematically investigated the adsorption states of alkali metal cations in 2:1-type swelling clay minerals via first-principles-based metadynamics simulations by fully utilizing supercomputers. We found that the characteristic adsorption state for light- and heavy-alkali-metal cations observed in tri-octahedral clay minerals (Fig.1-34) tends to disappear in di-octahedral ones, suggesting that particular clay minerals selectively adsorb Cs, in agreement with the experimental observations.
The present success in characterizing interlayer water and cations in clay minerals, which is difficult to achieve experimentally, enables us to promote the development of an economical, yet efficient, method for reducing the volume of waste soil.