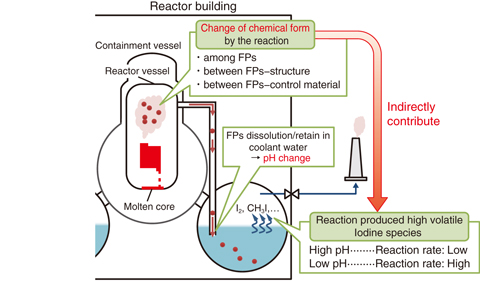
Fig.2-8 Chemical behavior of fission products during severe accidents
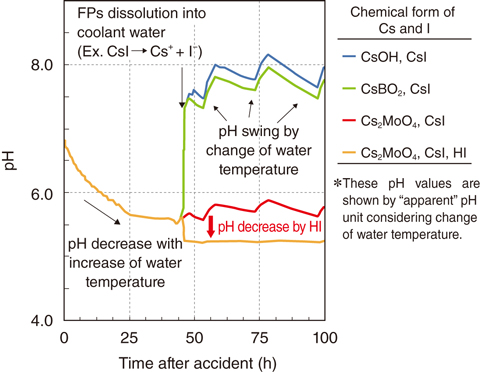
Fig.2-9 Calculated pH histories depending upon the chemical forms of Cs and I
Evaluation of public exposure at severe accident (SA) with core melt requires source term information for easily released fission product (FP) such as Cesium (Cs) and Iodine (I), including the mass and chemical species of FPs released into environment. Although the source term can be generally evaluated with SA analysis codes based on various models for physical/chemical phenomena, these results include uncertainties attributed to difficulty in understanding phenomena under the severe condition. One important phenomenon with a large uncertainty and impact on the accident analysis at the TEPCO’s Fukushima Daiichi NPS is the effect of boron (B) as a control rod material upon the chemical forms of Cs and I. The change of chemical form should affect the conversion of I ions dissolved in coolant water into highly volatile species (Fig.2-8), because the FP dissolution into coolant water can change pH. Chemical forms of decreasing pH are important because the reaction is accelerated, especially at lower pH.
Therefore, we estimated the chemical forms of Cs and I in gas phases that included B and other FPs based on thermodynamic equilibrium calculations and then evaluated pH considering the amount of FPs transported into the coolant water.
Consequently, it was found that CsI, CsOH, Cs2MoO4, and CsBO2 were formed with Cs and that CsI and HI were formed with I in the gas temperature range from 500 to 3000 K. In addition, the amount of CsBO2 and HI increased with the decrease in the amount of Cs2MoO4 and CsI in the case of lager amounts of B. Their effect on pH was dependent upon the chemical form of each species (Fig.2-9). CsOH or CsBO2 with CsI contributed to the increase in pH. Cs2MoO4 with CsI did not significantly change pH, whereas Cs2MoO4 with HI decreased pH. Considering thermodynamic equilibrium for systems including Cs and B, the range of thermal stability differed between Cs2MoO4 and HI. However, from the viewpoint of kinetics, HI produced at high temperatures can exist with Cs2MoO4 stabilized at low temperature because the degradation rate of HI is lower at low temperature. Thus, the results obtained in this study indicate that boron’s effect on pH is of significant importance to evaluate the amount of I released because B can enhance the production of HI as a contributor to pH decrease.
For future work, we will incorporate the static/dynamic evaluation function for chemical form into the THALES2 code to upgrade the source term evaluation technique.