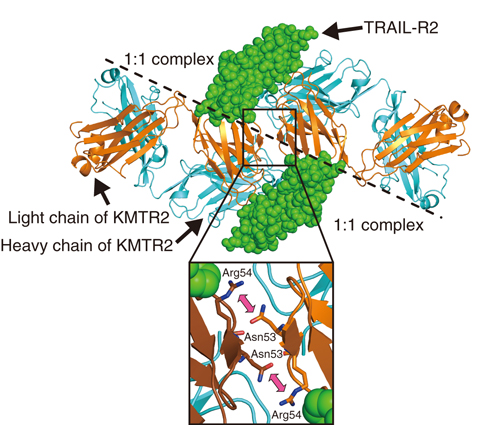
Fig.5-31 Functional unit for the reaction mechanism of KMTR2
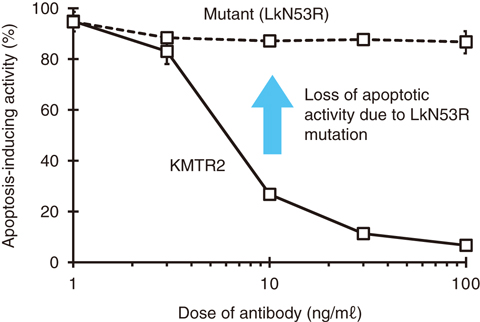
Fig.5-32 Apoptosis-inducing activity of KMTR2 and its mutant form
Tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL) induces apoptosis in various human cancer-cell lines but not in most normal human cells. TRAIL activates two distinct receptors, TRAIL-R1 and TRAIL-R2, both of which possess a death domain (DD) in their cytoplasmic tail that can interact with the apoptotic machinery. The fully human monoclonal antibody KMTR2, clusters TRAIL-R2 on tumor-cell surfaces and acts as a strong direct agonist for TRAIL-R2. Therefore, specifically targeting the apoptotic pathway with TRAIL-R2 using direct agonistic antibodies including KMTR2 may offer a novel therapeutic strategy for malignant tumors.
To investigate the mechanism of direct agonistic activity induced by KMTR2, the 1:1 complex structure of the extracellular region of TRAIL-R2 (ecTRAIL-R2) and a Fab fragment derived from KMTR2 (KMTR2-Fab) was determined to a 2.1-Å resolution by X-ray crystallography. The dimeric structure of two 1:1 KMTR2-Fab/ecTRAIL-R2 complexes rendered by crystallographic symmetry is shown in Fig.5-31. Two KMTR2-Fab molecules interacted with the second complementarity-determining region (CDR2) of the light chain via two-fold crystallographic symmetry, and consequently, two ecTRAIL-R2 molecules were situated in parallel within a C-terminal distance of 20 Å. It is believed that this adjacent configuration of the extracellular region resulted in access to each DD in the intracellular regions of the TRAIL-R2 molecules, leading to activation of a caspase-dependent apoptotic pathway. We assumed that Fab dimerization based on this two-fold symmetry was essential for the direct agonistic activity of KMTR2.
To confirm this assumption, we designed a mutant, replacing Asn53 with Arg in the light kappa chain of KMTR2 (LkN53R) to prevent dimerization at this site (close-up view in Fig.5-31). Because Asn53 in the CDR2 of the light chain was located near the two-fold axis between the two KMTR2-Fab fragments, the Asn53-to-Arg mutation should have introduced both steric hindrance and charge repulsion from the associations of four positive charges, two from Arg53 after mutation and two from Arg54.
Several biochemical experiments using KMTR2 and its mutant LkN53R were performed to confirm this assumption. Both flow cytometry and ELISA analyses clearly indicated that the Asn53-to-Arg mutation had little effect upon the binding activity of TRAIL-R2. In addition, both size-exclusion chromatography and confocal-microscopy techniques showed that the LkN53R mutation abolished the higher oligomerization. Furthermore, the LkN53R mutation resulted in a loss of its apoptotic activity (Fig.5-32) in conjunction with its caspase activation. These results indicate that strong agonistic activity induced by KMTR2, such as apoptotic signaling and tumor regression, can be attributed to TRAIL-R2 superoligomerization induced by the interdimerization of KMTR2, shown in Fig.5-31 as an essential functional unit.
The present study was accomplished in collaboration with the JAEA and the Kyowa Hakko Kirin Co., Ltd.