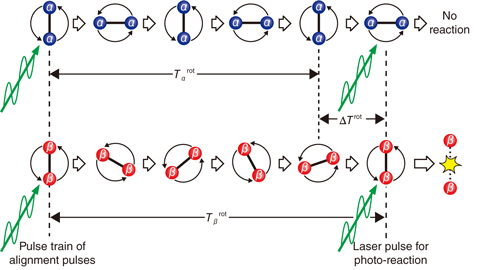
Fig.5-43 Diagram of laser-isotope separation utilizing control of molecular rotation
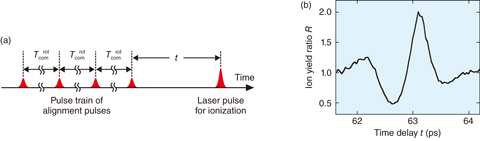
Fig.5-44 Experimental result of N2-isotope-selective ionization with a femtosecond-laser-pulse train
A conventional molecular-laser isotope-separation (MLIS) method uses isotope-selective vibrational excitation of isotopologues. The isotope selectivity originates from differences in the vibrational frequencies of the isotopologues. Because the frequency difference decreases as isotope mass increases, the isotope selectivity is significantly lowered when we apply this method to heavy isotopes.
We have proposed a new MLIS utilizing control of molecular rotation. According to this method, isotopologues are selected depending on their rotational periods (Fig.5-43). Femtosecond (fs) laser pulses with linear polarization (alignment pulses) create rotational wave packets in the isotopologues, which show an alignment condition just after the fs-pulse irradiation (the left side of Fig.5-43). The alignment condition disappears under time evolution of the wave packets and revives periodically with a rotational period of Trot. Trot of one isotopologue is different from that of the other. Additional fs-laser-pulse irradiation when one of the isotopologues aligns can induce isotope-selective photoreaction. Because the difference of Trot (ΔTrot) does not depend significantly upon the isotope mass, the isotopic selectivity of the new method is not significantly lowered for heavy isotopes.
To demonstrate the new method, a gas mixture of N2 isotopologues (14N2 and 15N2) was used. After a time delay t following pulse-train irradiation of four alignment pulses with the pulse interval being equal to the common rotational period of the isotopologues (Tcomrot = 15T14rot = 14T15rot), an additional laser pulse ionized the N2 isotopologues (Fig.5-44(a)). As shown in Fig.5-44(b), the ion-yield ratio R [= I(15N2)/I(14N2)] varies in the range of 0.49–2.00, depending on the time delay t. This result indicates that isotope-selective ionization can be realized by simply tuning t.
We expect that the new method will be applicable for separation of heavy isotopes, leading to advances in radioactive-waste management.
This research was partly supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Challenging Exploratory Research (No.23656594), and by the Consortium for Photon Science and Technology.