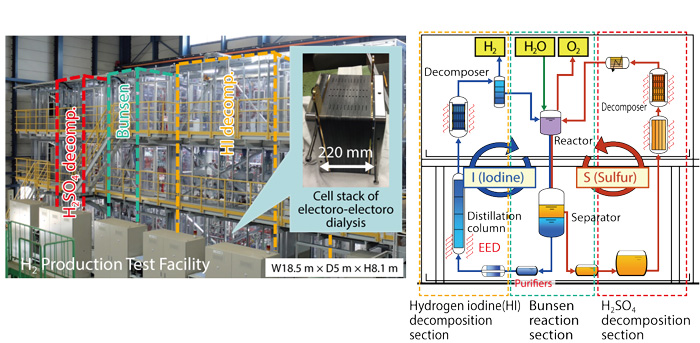
Fig.6-7 External view of the hydrogen-production test facility
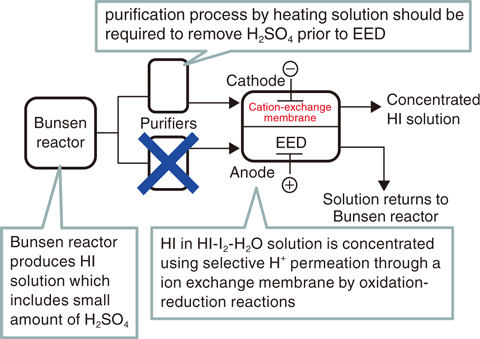
Fig.6-8 Production and concentration of HI Solution
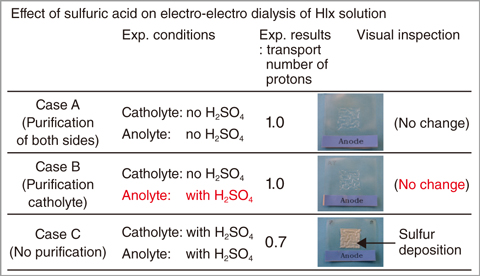
Fig.6-9 View of cation-exchange membrane after HI-concentration experiments using the EED device
We have pursued R&D on a thermochemical hydrogen-production iodine–sulfur process as a heat-utilization application for the high-temperature gas-cooled reactor. This chemical process uses chemical compounds of iodine and sulfur to split water to produce hydrogen by combining three chemical reactions. This process, which can harness heat for nuclear or renewable energy, is a promising next-generation hydrogen-production method that is independent of fossil fuels and that can provide energy security. One important task is to improve the thermal efficiency of hydrogen production.
Reduction of the heat for driving processes is an effective method of improving thermal efficiency. We focused on a purification operation for processing the Bunsen-reaction solution. Bunsen reaction, which is core chemical reaction in the iodine–sulfur process, produces two types of acids in HI-and H2SO4-rich solutions from H2O, I2, and SO2; the two acids separate into upper and lower phases with a clear boundary set by the liquid–liquid phase-separation phenomenon. The lower phase, which is rich in HI and I2, includes an impurity of H2SO4; this contamination probably causes HI concentration (an EED with a cation-exchange membrane, Fig.6-7) and HI distillation to occur as a harmful side reaction with the production of solid sulfur. Because of mutual solubility between the two phases, a certain amount of H2SO4 is solved into the lower phase; a purification process by heating of the solution is necessary to remove H2SO4. This purification causes an increase of heat input because of the heating requirement for solution vaporization.
While optimizations of the operating temperature for the purification process have been studied, we proposed a flow-rate-reduction method of the processed solution itself to reduce the required heat (Fig.6-8), based on the fact that H2SO4 turns into sulfur in the reduction environment. The solution after the purification should be concentrated by EED; the solution is fed to both channels of the EED device in the previous flowsheet; H2SO4 in the anolyte of the oxidation environment is therefore probably stable. If the method is workable, the required heat can be reduced by reducing the processing solution for the purification; moreover, the flowsheet can be simplified.
To validate the proposed method, we conducted concentration experiments of the HI solution including H2SO4 using an EED device. Results met our expectations; the sulfur was not produced in the anolyte; moreover, the contamination in the anolyte was not affected by the cell voltage or the membrane selectivity (Fig.6-9). We revealed that our proposal can save the input heat required for purification. An increase of the hydrogen-production thermal efficiency is approximately 10% of that of the previous method.