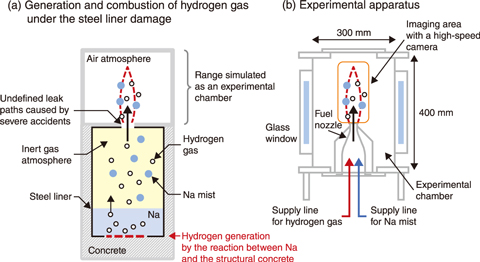
Fig.7-12 (a) Hydrogen generation when a steel liner is damaged and (b) the designed experimental apparatus
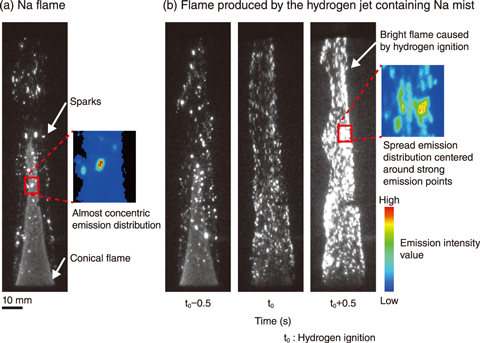
Fig.7-13 Observed flames: (a) Na and (b) hydrogen containing Na mist
Sodium (Na) used in sodium-cooled fast reactors (SFRs) burns in oxygen and can react with water to generate hydrogen gas. To prevent leaked Na from reacting with water in structural concrete, a steel liner is built inside a room where Na components or Na pipes are installed. In the event of severe accidents, however, a Na-concrete reaction caused by a damage to the liner would generate hydrogen gas and Na mist (fine droplets). When this hydrogen gas and Na mist are emitted into the room filled with air, hydrogen is considered to burn like a burner besides sodium combustion as shown in Fig.7-12(a). This combustion of hydrogen jets containing Na mist is a phenomenon specific to SFRs and is a different combustion mode from hydrogen deflagration due to hydrogen accumulated in the room. The ignition mechanism of hydrogen with a burning Na mist must be clarified to gain a better understanding of ignition risks of SFRs during severe accidents.
Therefore, a combustion experiment was performed to visualize the ignition process of hydrogen jets by high-speed and high-sensitivity imaging. A Na mist (about 15 g/m3) was produced from Na vapor heated at 750 ℃ in an inert gas atmosphere and was mixed with 10% hydrogen (260 ℃, diluted with an inert gas). The hydrogen gas and Na mist were then emitted into an experimental chamber containing 21% oxygen, which was designed to simulate a room filled with air (Fig.7-12(b)). Additionally, as a comparative experiment, only Na mist was emitted without hydrogen gas; here, the burning Na mist produced a conical flame, caused by combustion of invisible small mist, and many sparks scattered intermittently outside the conical flame, as shown in the monochrome image of Fig.7-13(a). With the inclusion of hydrogen, the Na flame was also formed; however, within 1 s, a new bright flame was produced that gradually covered the conical Na flame, as visible in Fig.7-13(b). Because this bright flame only appeared when the Na mist and hydrogen coexisted, the flame can be attributed to the ignition of hydrogen.
To focus on the change in brightness of flame from the time (t0) when this change started compared to the brightness of only Na flame, the spatial distribution of emission intensity was analyzed using a coloring method that maps the intensity values into assigned colors, shown in the small colored maps of Fig.7-13. In the sparks of the Na flame, the emission distribution was nearly concentric and localized around a strong emission point generated by the burning of each Na mist particle (Fig.7-13(a)). Once the hydrogen was ignited, the distribution spread across a larger area and contained the sparks (Fig.7-13(b)). This difference in emission distribution indicates that during the ignition of the hydrogen jet containing the Na mist, hydrogen was locally ignited around the burning Na mist particles. Future work will investigate the ignition conditions of hydrogen under various oxygen concentrations.
This study is part of the results conducted on “Development of Estimation Technology for Availability of Measure for Failure of Containment Vessel in Sodium Cooled Fast Reactor“ entrusted to University of Fukui, supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. JAEA was subcontracted by University of Fukui to carry out the research.
(Daisuke Doi)
<Previous: 7-4 | Next: 8 Research and Development on Fuel Reprocessing, Decommissioning, and Radioactive Waste Management>