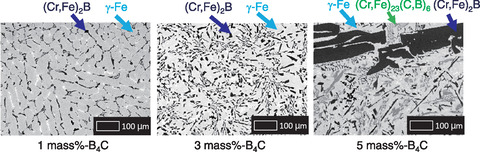
Fig.1-2 Backscattered electron images collected on cross sections of the prepared metallic fuel debris samples
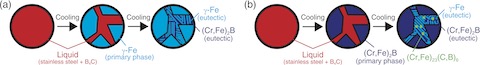
Fig.1-3 Solidification mechanism of metallic fuel debris
We at nuclear fuel debris science group aim to reveal the physical and chemical characteristics of the remaining fuel debris at TEPCO's Fukushima Daiichi NPS (1F), which is essential to develop secure and safe 1F fuel debris retrieval and decommissioning strategies. Although the fuel debris remaining in 1F is still unidentified at this time, it can be generally classified into three as follows:
• Metallic fuel debris composed mainly of stainless steel, boron carbide, and metallic zirconium,
• Oxide fuel debris consisting mainly of uranium and zirconium oxides, and
• Concrete-based fuel debris with a silicate matrix.
Boron carbide (B4C) used in the control rods of 1F causes eutectic melting reaction with metals to form metallic fuel debris. Therefore, it is presumed that a large amount of metallic fuel debris remains in 1F. Metallic fuel debris was, however, not generated in the past severe accidents (i.e., at Three Mile Island or Chernobyl) because of the difference in reactor type. Hence, the characteristics of the metallic fuel debris are mostly veiled even to date. In order to reveal the formation mechanisms of metallic fuel debris, this study performed high temperature reaction experiments by melting stainless steel and boron carbide, and prepared metallic fuel debris samples under various conditions.
Example backscattered electron images collected on cross sections of the prepared metallic fuel debris samples are shown in Fig.1-2. The (Cr,Fe)2B phase (i.e., a chromium-iron-boron intermetallic compound) was found to crystallize and grow with increasing B4C content. A summary of the solidification mechanism of the metallic fuel debris based on the results from our detailed analysis is shown in Fig.1-3. Here, γ-Fe (i.e., a metallic phase of iron) was the primary phase when the initial mass concentration of B4C was below 3 mass%, as shown in Fig.1-3(a). When the B4C mass concentration exceeded 3 mass%, however, (Cr,Fe)2B became the primary phase, and a third phase, (Cr,Fe)23(C,B)6, was also formed, as shown in Fig.1-3(b). This solidification mechanism can vary with the carbon, boron, and chromium concentration.
This research demonstrates how our research group is tackling the problems posed by fuel debris from the viewpoint of basic research.
This work was supported financially in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Early-Career Scientists (No.JP20K15209).
(Takehiro Sumita)
<Previous: 1 Research and Development Related to the Accident at TEPCO's Fukushima Daiichi NPS | Next: 1-2>