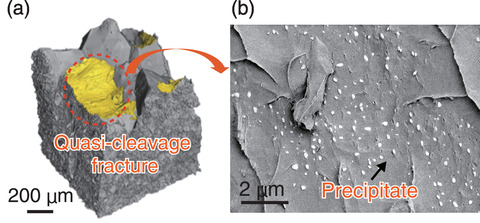
Fig.4-13 Experimentally observed fracture surface of an Al-Zn-Mg alloy
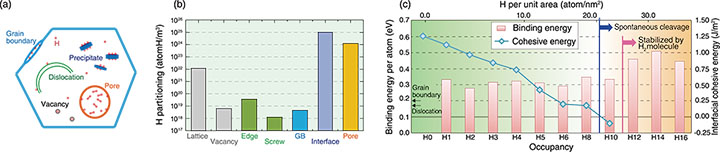
Fig.4-14 Hydrogen partitioning and spontaneous cracking
Aluminum alloys are considered the most feasible material to satisfy lightweight structural and industrial needs. Highly concentrated precipitates generated by age hardening play a dominant role in shaping the mechanical properties of aluminum alloys. It is commonly believed that the coherent interface between the matrix and precipitate does not contribute to crack initiation and embrittlement. High-strength lightweight aluminum alloys have been expected for a long time.
Therefore, we performed a three-dimensional observation technique and atomistic simulations to clarify the hydrogen embrittlement mechanism related to the quasi-cleavage fracture unique to Al alloys. Synchrotron X-ray imaging and scanning electron microscopy (SEM) with energy-dispersive X-ray spectroscopy analysis were used to capture the characteristic features of the fracture and, in particular, elucidate the relationship between the fracture and coherent surface planes of the η-phase precipitates.
The fracture surface obtained by synchrotron X-ray tomography corresponded to a brittle fracture, as shown in Fig.4-13(a). The crack propagated gradually and undulatory along various quasi-cleavage facets where hydrogen atoms were sufficiently trapped. Low-voltage SEM images on this surface are shown in Fig.4-13(b). Surprisingly, very dense white particles were observed, indicating that the fracture occurred along the Al-MgZn2 precipitate interface.
The binding energy between hydrogen atoms and various defect structures, such as vacancies, edge/screw dislocations, grain boundaries, and precipitates, were then investigated, as summarized in Fig.4-14(a). The theoretically estimated hydrogen partitioning (i.e., site occupancy) is provided in Fig.4-14(b). Site occupancy differed widely, reflecting the exponential contribution of the binding energies. Hydrogen partitioning was found at the interface of the precipitate and aluminum matrix. DFT calculations were performed again to explore the relationship between cohesive energy and occupancy at this interface; the resulting binding energy per atom and interfacial cohesive energy in terms of occupancy is shown in Fig.4-14(c). Despite the coherent interface, the binding energy did not decrease, even at maximum occupancy. Once occupancy exceeded a certain limit, hydrogen atoms tended to stably nucleate as hydrogen molecules. Thus, the interface cohesivity deteriorates significantly with increasing hydrogen occupancy, whereas hydrogen atoms can be stably trapped up to an extremely high occupancy that is equivalent to spontaneous cleavage.
Computational simulations are expected to aid in the design of alloys to serve industrial needs.
This study was supported by Japan Science and Technology Agency (JST) Strategic Basic Research Programs (CREST, JPMJCR1995), and Industry-Academia Co-creation Basic Research Program (Project 20100114).
(Tomohito Tsuru)