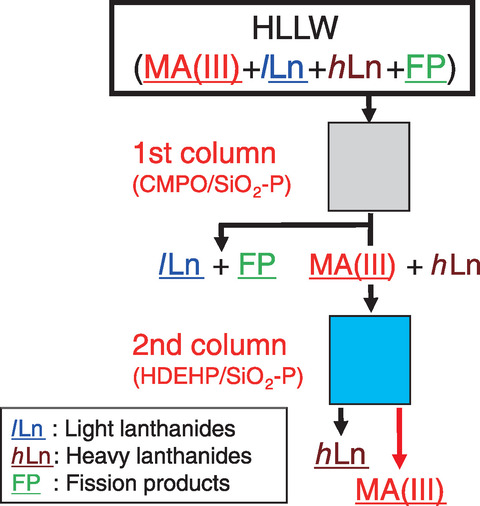
Fig.7-11 Improved MA(Ⅲ) recovery process
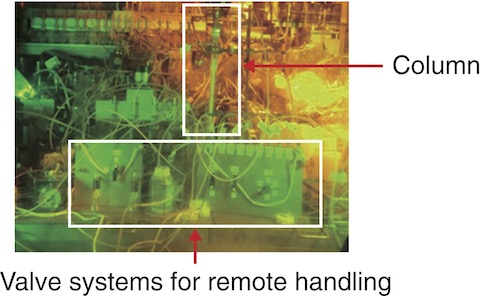
Fig.7-12 Experimental setup inside the hot cell
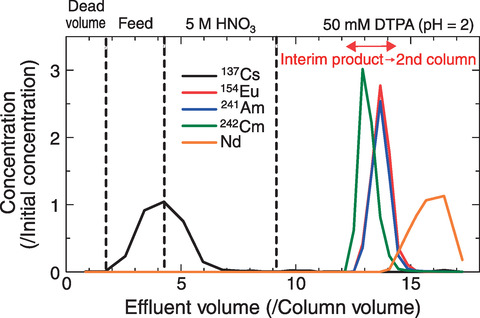
Fig.7-13 Elution curves obtained by the CMPO column
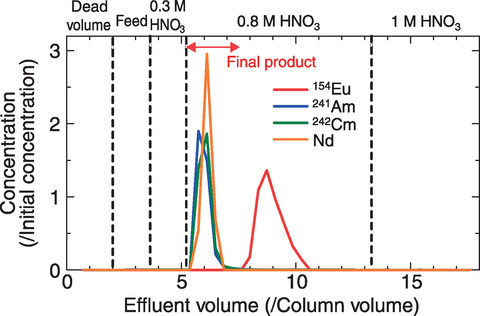
Fig.7-14 Elution curves obtained by the HDEHP column
Partitioning and transmuting minor actinides (MAs(Ⅲ)), including Am and Cm, may reduce the volume and radiotoxicity of radioactive wastes owing to their long half-life and heat generation natures. Many researchers worldwide have aimed to separate MA(Ⅲ) from high-level liquid wastes (HLLW) containing various metals. The main challenging task for selective MA(Ⅲ) recovery is their separation from trivalent lanthanides (Lns(Ⅲ)) because of the chemical similarity between MAs(Ⅲ) and Lns(Ⅲ) in solution.
Solvent extraction is a proven methodology that is commonly studied for MA(Ⅲ) separation worldwide. We have focused on developing an extraction chromatography technology that is based on the principles of solvent extraction but is expected to generate less secondary waste. This technology uses porous silica particles coated with a polymer (SiO2-P)-impregnating solvent to adsorb MAs(Ⅲ), thereby allowing MAs(Ⅲ) to be recovered from the HLLW through column separation. Our proposed two-step column operation uses CMPO and HDEHP extractants as adsorbents in the first and second columns, respectively. Here, MAs(Ⅲ) and Lns(Ⅲ) are recovered in the first column; MAs(Ⅲ) are then separated from Lns(Ⅲ) in the second column. Using the proposed method, MAs(Ⅲ) were separated from Ln(Ⅲ) successfully. However, the recovery ratio of Mas(Ⅲ) was relatively low (70%), and the product solution contained the complexing reagent used for MA(Ⅲ) elution, thereby requiring a separate removal step.
To improve MA(Ⅲ) recovery, therefore, we designed a separation process using the difference in affinity of Lns(Ⅲ) to CMPO and HDEHP, as shown in Fig.7-11, by focusing on the difference between light Lns (ILns: La-Nd) and heavy lanthanides (hLns: Sm-Gd). DTPA, which was used in the second column of the original method, was used in the first column of the modified one, and MA(Ⅲ) was expected to be recovered in a solution of pure nitric acid. The proposed methodology was then demonstrated on real HLLW, as shown in Fig.7-12, after minor modifications were obtained from column separation experiments using simulated HLLW. Using the proposed methodology, ILns and other fission products were removed in the first column, and MAs(Ⅲ) were then separated from hLns in the second column, as shown in Figs.7-13 and 7-14, respectively. Decontamination factors of Lns(Ⅲ) were larger than the target value 102 although Nd was not decontaminated by the second column. Overall, we successfully designed and demonstrated a MA(Ⅲ) recovery process that achieved over 90% MA(Ⅲ) recovery in nitric acid solution with high purity.
A part of this study was carried out through collaboration with the Shibaura Institute of Technology under the project “Optimization in adsorbent for MA(Ⅲ) recovery”.
(Sou Watanabe)
<Previous: 7-4 | Next: 8 Research and Development on Fuel Reprocessing, Decommissioning, and Radioactive Waste Management>