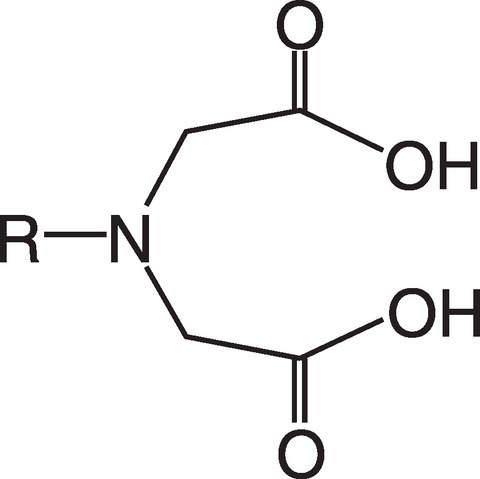
Fig.8-7 Structural diagram of iminodiacetic acid group
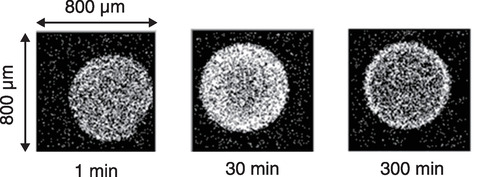
Fig.8-8 PIXE images of Zr adsorbed from simulated spent extraction solvent
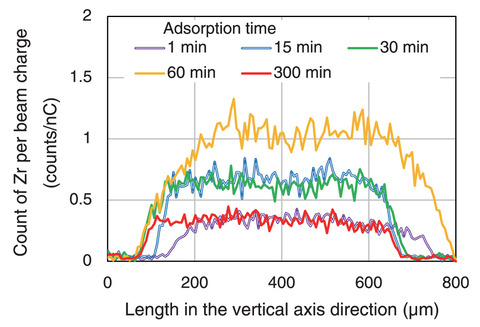
Fig.8-9 Zr detection strength of adsorbent (micro-PIXE analysis)
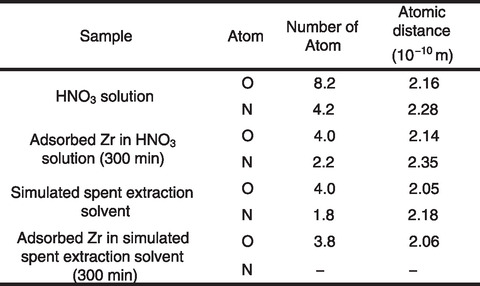
Table 8-1 Local structural parameters around adsorbed Zr (EXAFS analysis)
Radioactive waste liquids generated in nuclear facilities sometimes contain reactive chemical reagents in addition to radioactive elements. Some such waste liquids are currently stored inside nuclear facilities due to a lack of appropriate treatment processes. The spent extraction solvent, which mainly comprises tributyl phosphate (TBP) and normal dodecane, contains U and Pu and is generated during plutonium uranium redox extraction (PUREX) process. The spent extraction solvent is degraded by radiation from the extracted nuclear fuel material. Due to the risk of explosive substances being generated as products of radiolysis (e.g., hydrogen and nitrogen compounds), stabilization treatment must be performed promptly. Thus, nuclear materials must be removed from the organic liquid to ensure safety. One commonly used back extraction method used to recover nuclear fuel material from the spent extraction solvent uses a sodium carbonate solution; however, using this method generates a large amount of alkaline liquid waste. To design a recovery process that releases less secondary waste, we are focused on developing a process using an adsorbent containing iminodiacetic acid (IDA) functional groups, the structure of which is shown in Fig.8-7.
Here, we investigated the complex formation reaction of an IDA-type adsorbent with simulated nuclear fuel materials in the spent extraction solvent. The simulated spent extraction solvent was prepared by diluting TBP and dibutyl phosphate (DBP), which is produced by radiolysis or hydrolysis of TBP, with normal dodecane. Subsequently, a nitric acid (HNO3) solution containing Zr was mixed with the same solvent to load Zr onto the solvent, where Zr was used to simulate Pu. As a control, the HNO3 solution was also used as a feed solution to load Zr onto the adsorbent from the aqueous solution. The IDA-type adsorbent (CR11, Mitsubishi Chemical) was contacted with the liquid in a vial and shaken for 1 to 300 minutes. The distribution of Zr and the chemical form of the complex were then analyzed using micro-particle induced X-ray emission (PIXE) and extended X-ray absorption fine structure (EXAFS), respectively, to clarify the adsorption reaction of the IDA-type adsorbent.
The resulting distribution of Zr adsorbed on the adsorbent and Zr detection strength of adsorbent by micro-PIXE analysis are summarized in Figs.8-8 and 8-9, respectively. Micro-PIXE analysis revealed that Zr was distributed inside the adsorbent within one minute. The local structural parameters around the adsorbed Zr are detailed in Table 8-1. EXAFS analysis suggested that the chemical species of Zr trapped in adsorbent from the HNO3 solution and simulated spent extraction solvent were ZrO(NO3)2 and ZrO2+, respectively. Since the IDA-type adsorbent can form complexes with Zr atoms in the solvent, this suggests that an IDA-type adsorbent can be used to treat spent solvent.
The micro-PIXE and EXAFS analyses were performed at Takasaki Ion Accelerators for Advanced Radiation (TIARA) of National Institutes for Quantum and Radiological Science and Technology and the BL11S2 beamline of Aichi SR, respectively.
(Yoichi Arai)