Table 1-1 Examples of simulated debris samples prepared in this study
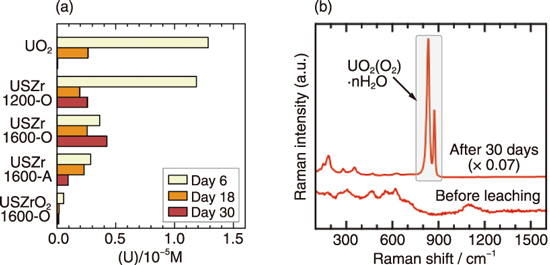
Fig.1-3 (a) U concentrations in the leachate and (b) Raman spectra of uranyl peroxides formed on USZr1600-O by immersion in an aqueous H2O2 solution
The fuel debris generated in the accident at TEPCO’s Fukushima Daiichi Nuclear Power Station remains in the reactors, and substantial time and effort will be required for the retrieval of the debris. The debris has been most likely exposed to water since the accident. Contact with water results in the degradation of the debris matrix due to the radiolysis of water. Water radiolysis generates oxidants such as hydrogen peroxide (H2O2), which can oxidize uranium to hexavalent U(VI). According to previous studies on uranium (IV) oxide (UO2) and spent fuels, U oxidation proceeds at the interface with water, and the matrix gradually dissolves because U(VI) has a higher water-solubility than U(IV). Hence, to examine the possible degradation processes of fuel debris, we performed leaching experiments using simulated fuel debris.
The simulated debris samples were prepared from powders of UO2, stainless steel (SUS 304), and zirconium metal (Zr) or oxide (ZrO2) by heat treatment under various conditions(Table 1-1). After analyzing the phase composition of the samples, the simulated debris samples were immersed in an aqueous H2O2 solution for up to 30 days. H2O2 was added because it is the water radiolysis product that has major impact on U oxidation. After certain periods of immersion, the samples were analyzed by Raman spectroscopy, and chemical analysis of the leached elements was performed.
The analysis of the leached elements showed significant dissolution of U from the samples. The reaction of H2O2 concurrently induced the precipitation of uranyl peroxides, UO2(O2)·nH2O(n = 2 or 4). Because of these two processes, the dissolved U concentration once increased and then decreased with leaching time (Fig.1-3 (a)). The formation of uranyl peroxides was clearly confirmed by the Raman spectroscopy results (Fig.1-3 (b)). These results demonstrate that uranyl peroxides are the possible alteration products of fuel debris from the H2O2 reaction.
In contrast, the sample in which formation of a U-Zr oxide solid solution proceeded to a remarkable degree (USZrO21600-O) showed much less U dissolution and no Raman signal of uranyl peroxides. This finding indicates that formation of the oxide solid solution of Zr with UO2 improves the durability of fuel debris against the H2O2 reaction.
This work was supported by JAEA Nuclear Energy S&T and Human Resource Development Project through concentrating wisdom (Grant number JPJA18P18071886).
(Yuta Kumagai)
<Previous: 1 Research and Development Related to the Accident at TEPCO’s Fukushima Daiichi NPS | Next: 1-2>