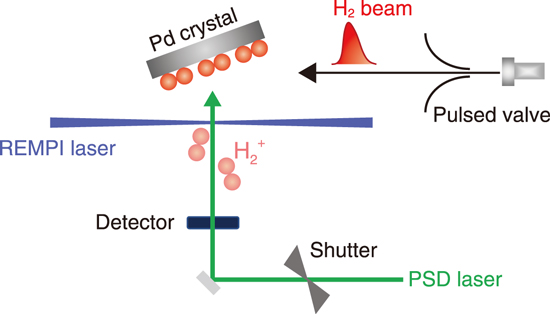
Fig.3-10 Development of a measurement method for surface adsorbed H2 ortho-to-para (o-p) conversion time based on a combination of a pulsed molecular beam, photo-stimulated desorption (PSD), and resonance-enhanced multiphoton ionization (REMPI) techniques
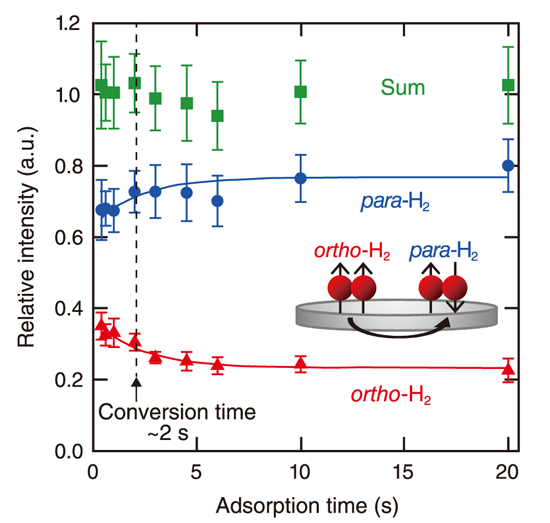
Fig.3-11 Time evolution of the photodesorbed H2 intensities for o-H2, p-H2, and their sum
Hydrogen is promising as a clean energy source that does not produce greenhouse and harmful gases. Two nuclear-spin modifications exist for H2: ortho-H2 (o-H2) and para-H2 (p-H2). When liquefying and storing hydrogen, it is necessary to para-hydrogenate the o-H2 before liquefaction because liquefying hydrogen gas containing o-H2 mixed in it causes heat generation due to the rotational energy of o-H2. This phenomenon is called the boil-off problem. Although it is known that ortho-to-para (o–p) conversion occurs on solid surface, the conversion is not efficient. In addition, the detailed conversion mechanism still remains unclear at present. Therefore, unraveling the conversion mechanism and developing a highly efficient conversion catalyst based on this knowledge are crucial to utilize hydrogen as an energy source.
In most of the past studies on o–p conversion on solid surfaces, H2 in the physisorption state was focused. However, it is known that a unique molecular chemisorption of H2 occurs on some stepped surfaces in addition to the typical adsorption scheme of physisorption. The previous studies indicate that the o–p conversion of molecularly chemisorbed H2 is faster than that of physisorbed one. However, because of the limited time resolution of the measurement techniques used, it was difficult to probe the o–p conversion. Thus, a direct measurement of the conversion time was not possible. Against this background, we recently developed a new method to determine the o–p conversion time by using a combination of a pulsed molecular beam, photostimulated desorption, and resonance-enhanced multiphoton ionization techniques. In this method, the time resolution was improved by about two orders of magnitude (Fig.3-10). We successfully tracked the change in the H2 nuclear-spin state and experimentally determined the o–p conversion time of the molecularly chemisorbed H2 on palladium (Pd) (210) face at a surface temperature of 50 K to be about 2 s (Fig.3-11). This value is 2–3 orders of magnitude smaller than those reported for physisorption systems, implying that the o–p conversion occurs rapidly. H2 molecularly chemisorbs on top of the step-edge Pd atom. This fast conversion of H2 in the molecular chemisorption state is a result of the strong interaction between H2 and the Pd substrate which is ascribed to the unique step surface structure. The present study indicates the importance of the surface structure in o–p conversion and is expected to lead to the design of highly efficient conversion catalysts.
This research was funded by JSPS KAKENHI Grants No. JP17H01057, JP18H05518, and JP20K05337, and the Reimei Research Program (JAEA). H. U. is partially supported by MEXT, Leading Initiative for Excellent Young Researchers.
(Hirokazu Ueta)