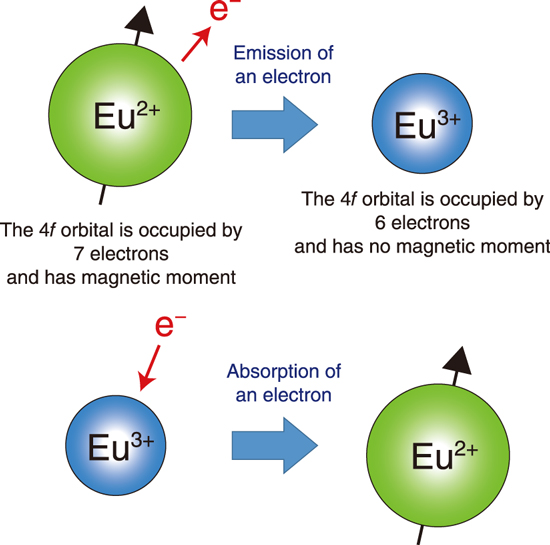
Fig.5-14 Valence instability of Eu-based compounds
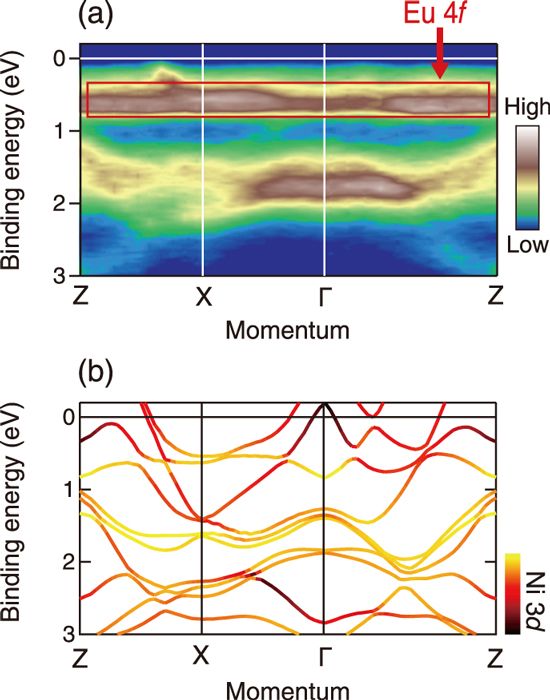
Fig.5-15 Experimental and calculated band structures of EuNi2P2
Rare-earth-based compounds possess various intriguing properties such as superconductivity and thus have garnered much attention in the field of strongly correlated electron physics. Moreover, these compounds have been considered as potential candidates for magnetic devices as the rare earth atoms have large magnetic moments. The physical properties of the rare-earth-based compound are accounted for by the 4ƒ electrons of the rare earth atoms. The 4ƒ electrons acquire itinerant properties through hybridization with other electron orbitals and form energy bands and fermi surfaces. This hybridization is the origin of the various magnetic orderings and superconductivities of rare-earth-based compounds. However, a relatively small 4ƒ binding energy is required to hybridize with other electron orbitals, and therefore, observations of superconductivity have been reported only for Ce- and Yb-based compounds so far. On the other hand, in recent years, it has been recognized that for some Eu-based compounds, the hybridization effect on the 4ƒ electrons becomes important and leads to various unusual properties.
It is well known that most rare earth atoms are nearly trivalent states in intermetallic compounds. However, some Eu-based compounds show a valence instability between the trivalent Eu3+ and divalent Eu2+ states because of the small energy difference between these states. As shown in Fig.5-14, this valence fluctuation corresponds to an instability of the number of 4ƒ electrons. Because of this instability, the 4ƒ electrons acquire a mobility that enables them to move throughout the crystal and govern the electronic and physical properties of Eu-based compounds. Although no Eu-based superconductor has been discovered until now, it has been reported that some Eu-based compounds exhibit heavy electron behavior in which the effective electron mass is strongly enhanced because of the electron correlation effect.
EuNi2P2 is known as the first heavy-electron system among the Eu-based compounds. In this study, we have performed angle-resolved photoemission experiments on EuNi2P2 at the JAEA beamline BL23SU at SPring-8. In angle-resolved photoemission, the energy and angular distributions of the photoelectrons were measured to directly observe the band structure of the materials.
Fig.5-15(a) shows the experimental band structure determined by angle-resolved photoemission. We observed a flat band structure originating from the Eu 4ƒ orbitals. The flatness of the bands reflects a large effective electron mass, and thus, this band shape seems to be the origin of the heavy electron behavior in EuNi2P2. A comparison with the calculated band structure (Fig.5-15(b)) shows that Eu 4ƒ electrons hybridize with other orbitals such as Ni 3d. This result confirms that Eu 4ƒ electrons interact with other electron orbitals and contribute to the formation of the band structure because of valence instability. Moreover, we confirmed the presence of Eu 4ƒ at the Fermi level. This means that the electronic and thermodynamic properties of this compound are markedly affected by the Eu 4ƒ-derived heavy electrons as these properties are usually governed by electron excitations at the Fermi level.
The present result provides an important clue for understanding the mechanism of formation of heavy electrons in Eu-based compounds. We hope that Eu-based superconductors will be discovered in future experimental studies. Such a discovery will further expand the research field of Eu-based compounds.
(Ikuto Kawasaki)
<Previous: 5-6 | Next: 6 HTGR Hydrogen and Heat Application Research>