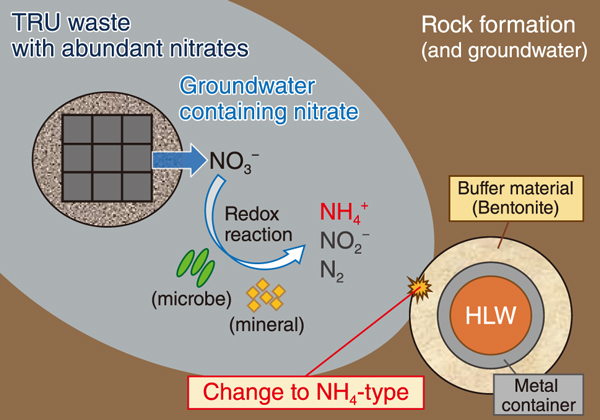
Fig.1 Possible event after co-location disposal of high-level radioactive waste (HLW) and transuranic (TRU) waste (considering the presence of NH4+)
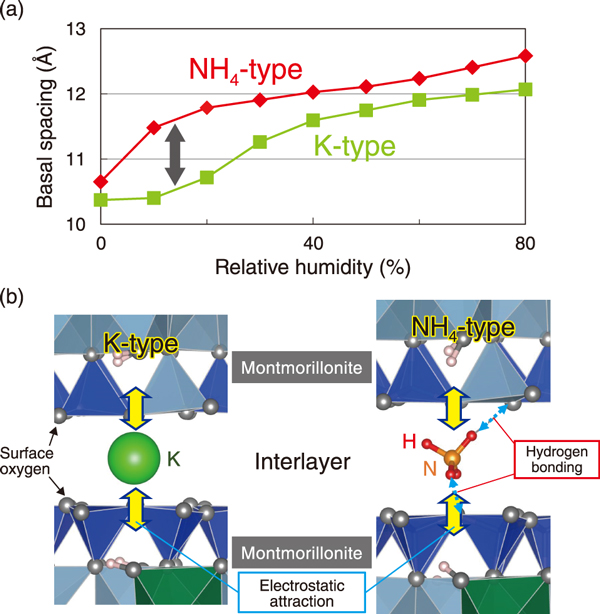
Fig.2 Behavior of interlayer cations on K-type and NH4-type montmorillonite
Transuranic (TRU) waste, generated from fuel reprocessing in the nuclear cycle, is planned to be disposed in co-location with high-level radioactive waste (HLW) at great depths under the ground. These wastes have different characteristics and may interact each other. As part of TRU waste contains abundant nitrates, NO3- can leach out and migrate in the geological environment while undergoing transformation of its chemical form.
In geological disposal, HLW is planned to be packed into the metal container (called the overpack), surrounded by buffer material (mostly bentonite), and placed into a stable rock formation. Owing to its high expandability, low permeability and absorptivity, the buffer material has potential for sealing of water and retarding radionuclide migration. These properties originate from the layered silicates, namely montmorillonite, but its performance may be altered by interlayer cations.
In the case of co-location disposal of HLW and TRU, NO3- in the leachate from TRU waste can be transformed to NH4+, and it can change the interlayer cations of montmorillonite to NH4+ after contact with buffer material in HLW (Fig.1). However, the characteristics of the NH4-type montmorillonite has hardly been studied. In this study, the expandability of the NH4-type montmorillonite was investigated by performing an X-ray diffraction (XRD) to measure the interlayer spacing under relative humidity control. We compared the behavior of this type of montmorillonite with that of the K-type montmorillonite, which has been well studied and is expected to exhibit an expansion behavior similar to that of the NH4-type. In addition, molecular dynamics (MD) simulation was conducted to investigate the behavior of cations at the montmorillonite interlayers and expansion mechanisms.
The XRD results show that compared to the K-type montmorillonite, the NH4-type has larger interlayer spacing in the dry state, and it expands at lower relative humidity (Fig.2(a)).
The MD simulations indicated that interaction between the interlayer cations and montmorillonite was electrostatic attraction in the case of the K-type montmorillonite, while it was electrostatic attraction and hydrogen bonding (between NH4+ and surface oxygen) in the case of the NH4-type (Fig.2(b)). In the K-type montmorillonite, K+ are packed into the surface cavity of montmorillonite, thereby reducing the interlayer spacing. However, in the case of the NH4-type, hydrogen bonding poses hinders such close packing, resulting in a larger interlayer spacing than in the K-type in the dry state. Consequently, water molecules can easily enter the interlayers of NH4-type montmorillonite, making it expand.
Considering that the buffer material is in contact with groundwater containing abundant NH4+ and that the montmorillonite changes to the NH4-type, the results imply that deterioration of swelling property of the buffer material may be smaller than in the case of the K-type.
This study was conducted in collaboration with Hokkaido University, and supported by METI (Grant number JPJ007597), as part of “The Project for Development on Evaluation and Validation Geological Disposal System (FY2013-2017).”
(Ryohei Kawakita)