We have examined the technology needed for a hydrogen production system by steam reforming of natural gas as a heat utilization system coupled to a High-Temperature Gas-Cooled Reactor (HTGR). Tritium (T), which is a radioactive isotope of hydrogen, is produced in the core of an HTGR. It is well known that hydrogen isotopes, hydrogen (H), deuterium (D), and tritium, permeate through solid metals. Thus, tritium produced in the core tends to permeate through heat transfer pipes of heat exchangers and catalyst pipes of a steam reformer in the H2 production system. Further, it is probable that the tritium will mix with the product hydrogen. To minimize the amount of permeated tritium, we have experimentally investigated the phenomenon of tritium permeation through a catalyst pipe when a large amount of H2 is present in the pipe.
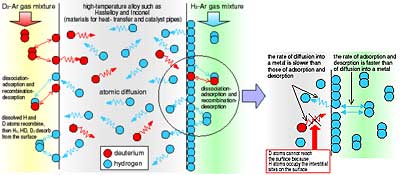
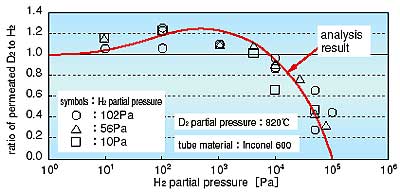
In the experiment, deuterium and hydrogen were used instead of tritium and hydrogen. Fig. 2-10 shows counter permeation of D2 and H2 through atoms in alloys such as Hastelloy or Inconel. Deuterium and hydrogen molecules will dissociate and the atoms will adsorb on the metal surface. The D and H atoms will then diffuse into the metal. Deuterium and hydrogen atoms diffuse in proportion to the concentration gradients of each. When these permeated atoms reach the opposite surface of the metal, both the D and H atoms will tend to recombine and desorb to the fluid phase. It is assumed that the rate of adsorption and desorption on the surface is faster than the rate of atomic diffusion (permeation) in the metal and that the partial pressure of H2 is much higher than that of D2. As H2 adsorbs and desorbs repeatedly on the surface containing mostly H atoms, the D atoms permeating through the metal cannot reach the surface because most interstitial sites on the surface are occupied by H atoms. Thus, little deuterium can recombine and desorb to the fluid phase (Fig. 2-11). From the results obtained in the experiment and analysis regarding the effectiveness of the H2 partial pressure on the amount of permeated D2, it is likely that the amount of permeated D2 to counter permeated D2 decreases with increasing H2 partial pressure. On the other hand, it also clear that the amount of permeated D2 increases slightly when the D2 partial pressure is near the H2 partial pressure (Fig. 2-12).
From this study, it was found that the tritium permeated through the catalyst pipe in the steam reformer decreases with increases in the partial pressure of the H2 produced in the steam reformer. Thus, the amount of tritium mixed in the product H2 may be minimized without tritium permeation countermeasures in H2 production systems coupled to HTGRs. |