Helium (He) remains in a liquid state even at very low temperatures, owing to its strong quantum nature. It is quite interesting to investigate chemical reactions in such a unique quantum medium because of the possibility of finding unknown chemical species as well as new synthetic pathways for molecules. From the theoretical point of view, it is generally very difficult to apply exact quantum-mechanical methods to such a many-atom chemical system.
We have recently developed a new approximate hybrid method, in which solute motions are described with a quantum wave-packet method, while solvent motions are treated by the path-integral centroid molecular dynamics method. This method has been applied to a fundamental chemical system: Photodissociation of a molecule in a He cluster. Using this computational technique, we can easily perform real-time simulations of the dynamics of chemical reactions involving many quantum particles.
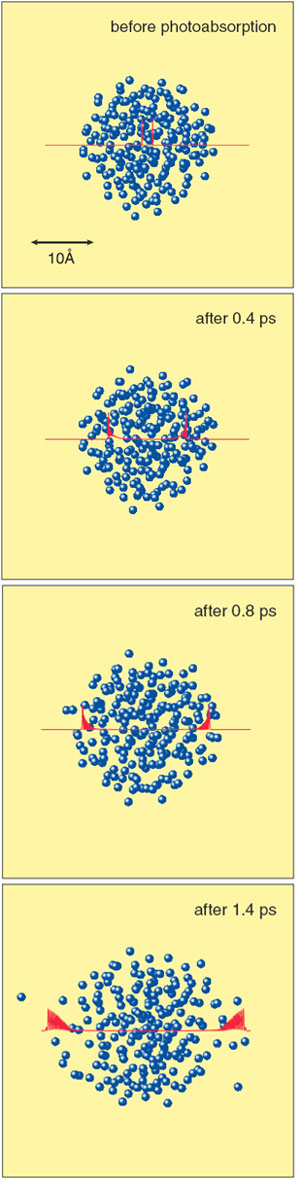
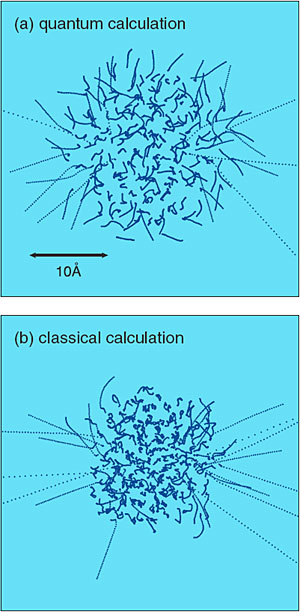
Fig. 7-12 shows simulation results for the dissociation dynamics of a chlorine (Cl) molecule embedded in a He cluster irradiated by a short laser pulse. Fig. 7-13 compares the quantum results with the corresponding classical results. In the quantum simulation, it is seen that the available energy generated in the course of the dissociation is quite rapidly converted into the translational energies of whole He atoms. We note that this unique feature, which occurs purely as a result of the quantum nature of He atoms, is nicely reproduced by our new hybrid method. We believe that this new method is destined to play an important role in the theoretical understanding of chemical reactions in low-temperature quantum media.
|