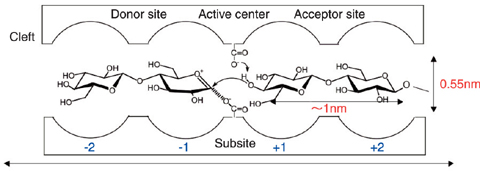
Fig.6-8 Schematic illustration of a so-called cleft, a specific reaction site in an enzyme
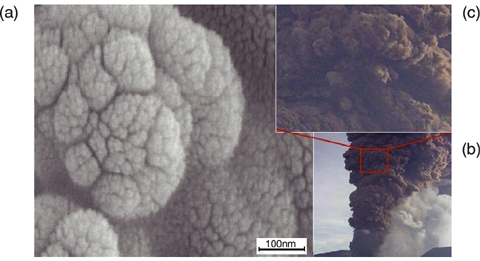
Fig.6-9
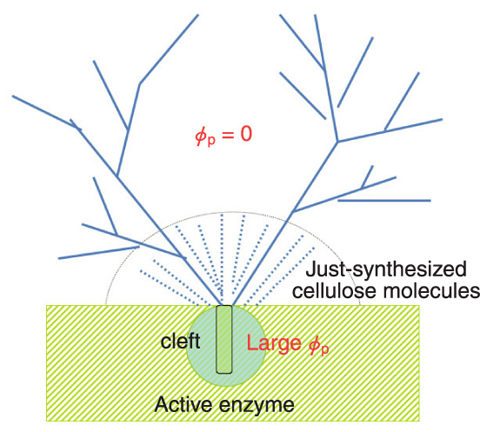
Fig.6-10 Schematic diagram showing the interface between the enzyme aggregate (the shaded green part) and the reaction medium
Cellulose, one of the important naturally made polymers, is biosynthesized at specific reaction sites in plants and bacteria. Synthesized cellulose molecules self-organize in-situ into crystalline fibrils. The specific reaction site is the so-called cleft found in active enzymes (Fig.6-8). Artificial syntheses of cellulose have been tried since 1941, based on traditional organic chemistry approaches, but none of them has succeeded. Kobayashi et al. first reported a successful synthesis based on enzymatic polymerization using cellulase as the catalytic enzyme and β-cellobiosyl fluoride as a substrate monomer (J. Am. Chem. Soc., vol.113, 1991, p.3079-3084).
In order to gain basic understanding of complex bioactivities concerning the cellulose synthesis and its self-organization into the cellulose fibrils, we attempted to explore the simple system employed by Kobayashi et al. using the time-resolved small angle neutron scattering spectrometer at the JRR-3 research reactor in JAEA. We elucidated for the first time in the world the following facts. (1) The enzyme molecules form aggregates having sizes greater than 200 nm in the reaction medium. (2) 1 g of active enzymes creates about 14 kg of cellulose during the whole reaction time, which means that one active enzyme creates about 5 cellulose molecules per second. (3) A large number of cellulose molecules, which have sprung out from the cleft assemble themselves into aggregates in the reaction medium and end up completely wrapping the enzyme aggregates. The surface of the cellulose aggregates (Fig.6-9(a)) has a self-similar roughness characterized by surface fractal structure with fractal dimension of 2.3 over an extremely wide length scale ranging from 30 nm to 30 µm, quite unique among the manifestations of fractal geometry. This surface structure is quite analogous to that formed by fumes from a volcano crater (Fig.6-9(b), (c)), except for the difference in length of a factor of 109 . The similarity of the two patterns shown in Fig.6-9 together with the above fact (2) help us to intuitively understand the enormous energy that the enzymatic reaction potentially has.