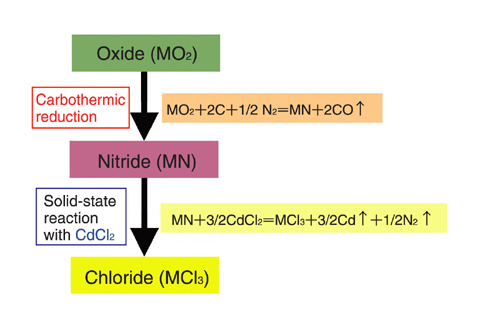
Fig.7-12 Outline of the method for the synthesis of MA chlorides
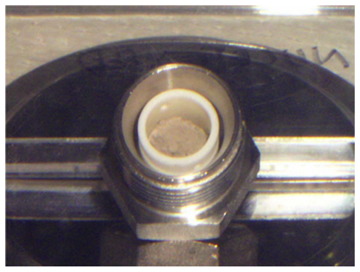
Fig.7-13 Appearance of the synthesized Am chloride (AmCl3)
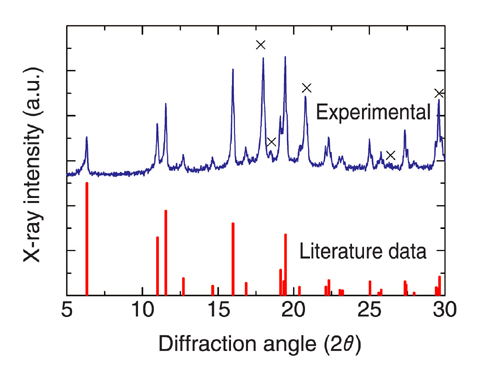
Fig.7-14 The X-ray diffraction pattern of the synthesized Am chloride (AmCl3)
In the advanced nuclear fuel cycles, minor actinides (MA: Np, Am, Cm), which are classified as high level wastes in the current nuclear fuel cycle, are to be recycled to reduce the burden of waste disposals. R&D of pyrochemical processes using molten salts as a solvent for treating MA-bearing fuels is underway. The pyrochemical processes are expected to be suitable for the treatment of spent nuclear fuels with high radioactivity and decay heat, and to have some advantages over hydrochemical processes in proliferation resistance, compactness, and economy. To develop the pyrochemical processes, understanding of MA behavior in molten salts is necessary, but few data are available. In order to obtain such data precisely, we installed a hot facility to handle MA chlorides in an inert gas atmosphere, because MA chlorides easily react with moisture and oxygen in air. A new method for the synthesis of MA chlorides on a gram scale without the use of HCl or Cl2 gas, which are used conventionally, was needed because these gases can corrode the materials of the hot facility.
We developed a method for the synthesis of MA chlorides from MA oxides, which are relatively easy to obtain, without the use of corrosive gasses. This method consists of two steps. First, nitrides are synthesized from the oxides by carbothermic reduction. Then, chlorides are synthesized by the solid-state reaction of the nitrides with cadmium chloride (CdCl2) (Fig.7-12). We succeeded in synthesizing high purity MA chlorides including americium trichloride (AmCl3) (Fig.7-13, Fig.7-14) in the Module for TRU High Temperature Chemistry (TRU-HITEC) maintained with argon gas. The solid-state reaction of the nitrides with CdCl2 is suited for synthesis not only of MA chlorides but also of other chlorides with high purity, because reagents containing oxygen are not used; they can easily react with chlorides to form stable by-products such as oxides.
Our experiments using the prepared MA chlorides are elucidating the behavior of MA in molten chlorides and the behavior of americium during the electrolysis of americium nitride in molten chlorides. We will continue to study on the behavior of MA in pyrochemical processes.
This study was carried out within the collaborative research program of TRU behavior in pyrochemical processes with Tohoku Electric Power Company, Tokyo Electric Power Company and The Japan Atomic Power Company.