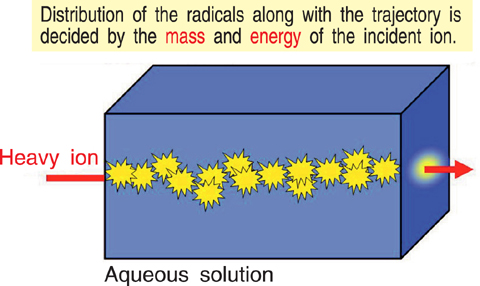
Fig.4-7 Radicals generated along heavy ion trajectory in water
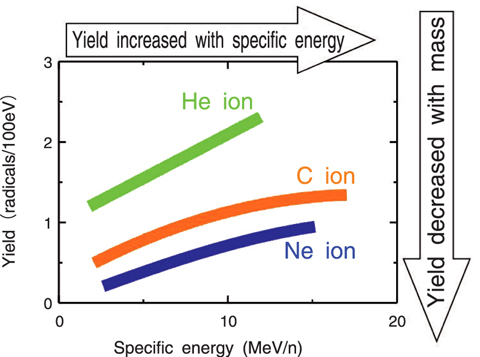
Fig.4-8 Dependence of yield of hydroxyl radicals on specific energy and mass of incident ion
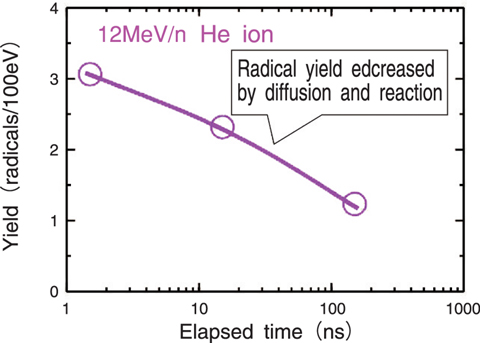
Fig.4-9 Yield of hydroxyl radicals decreased with elapsed time just after helium ion irradiation
High energy heavy ions are used for plant breeding and cancer treatment. The heavy ion can produce highly reactive species (e.g. hydroxyl (OH) radical) with a different distribution from that of conventionally used radiation, such as γ-rays, in a cellular tissue, i.e. an aqueous solution, as shown in Fig.4-7. Although the characteristics of the heavy ion irradiation effects can be explained in terms of LET (linear energy transfer) in many cases, one cannot explain the spatial distribution of the reactive species using LET. Moreover, the reactive species diffuse and react with time. Since the OH is considered the most important basic radical for radiation chemistry research, we estimated the yield of the OH radicals based on the mass and energy of the incident ion, and the elapsed time.
We irradiated aqueous phenol solution with heavy ions, changing the mass and energy systematically, and estimated the OH radical yield from the yield of reaction products of phenol. As a result, the yield of the OH radicals increased with the specific energy in the case of each ion, but decreased with the mass of the ion at the same specific energy as shown in Fig.4-8. The yield decreased with elapsed time (Fig.4-9). These results can be derived from the initial distribution of the reactive species around the heavy ion trajectory, and subsequent diffusion and reaction of the radicals.
Presently, we are constructing a time resolved optical measurement system. We will thereby increase the reliability of data about the radical yield, and observe directly reactions of radials with biomolecules.