Fig.8-3 Solvent degradation
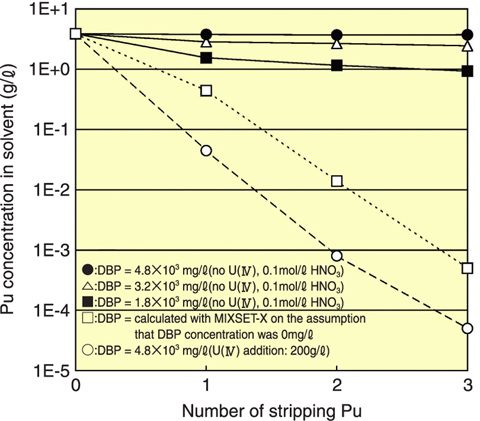
Fig.8-4 Pu concentration after stripping Pu in solvent
The reprocessing process in Tokai Reprocessing Plant (TRP) is PUREX process, using nitric acid and a solution of 30vol% TBP in n-dodecane.
TBP is degraded to DBP by radiolysis or hydrolysis etc. (Fig.8-3) Radiolysis depends on calorific value and hydrolysis with an ion catalyst depends on Pu concentration. We need to consider solvent degradation by the alpha rays from Pu because Pu concentration is high in the Pu purification cycle.
The Fugen spent fuel (MOX fuel) has been reprocessed at TRP since 2007. The amounts of Pu-238, Pu-240 and Pu-242 in MOX fuel are more than in LWR spent fuel. Pu-238, Pu-240 and Pu-242 are alpha ray emission nuclides. Therefore, it is thought that solvent degradation takes place more easily when MOX fuel is reprocessed.
We sampled solvent from contactors at Pu purification cycle when MOX fuel was reprocessed in TRP, and we evaluated the alpha rays emitted from Pu-degraded solvent by measuring Pu and DBP concentration in solvent. The results are as follows.
(1) Considering that total DBP production rate depends on calorific value and Pu concentration, total DBP production rate is calculated as follows:
T=51.2W + 0.06[Pu] + 0.1
Where T[mg/L・h] is total DBP production rate, W[W/L] is calorific value per unit volume and [Pu][g/L] is Pu concentration. W[W/L] corresponds to energy absorbed into solvent and is calculated from Pu concentration and Pu isotopic composition.
This formula enabled us to estimate DBP concentration in the Pu purification cycle from process parameters.
(2) The Pu stripping efficiency worsens when the DBP density is high, but we confirmed that there was no problem efficiently stripping Pu from solvent when U(IV) was used as the reduction reagent (Fig.8-4).
(3) We obtained the DBP concentration profile during the Pu purification step when the Fugen spent fuels were reprocessed in TRP.
In fact, we experienced a twenty day stoppage of the Pu purification cycle in TRP, during which time Pu remained in the solvent. After restarting Pu purification, we had no problem with stripping Pu from solvent, though DBP concentration was increased up to about 2.2×103mg/L. From the results of our investigation, we think the reason for this is that U(IV) was used as the reduction reagent for stripping Pu from solvent in the Pu purification step.
These results will be utilized as basic data for research into reprocessing MOX spent fuel.
<Previous: 8 Nuclear Fuel Cycle Technological Development | Next: 8-2 >