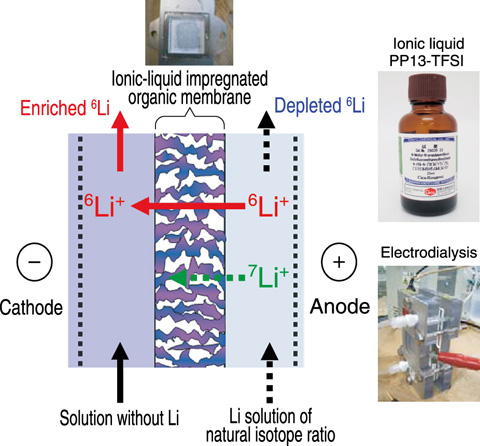
Fig.4-24 New lithium isotope separation technique using an ionic liquid
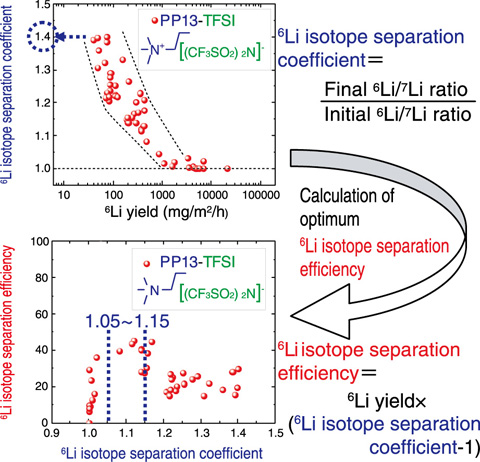
Fig.4-25 6Li isotope separation coefficient and efficiency
Tritium needed as a fuel for fusion reactors is produced via neutron capture by lithium-6 (6Li). However, natural Li contains only about 7.6% 6Li, and enrichment of 6Li up to 40∼90% is required for adequate tritium breeding in fusion reactors.
The amalgamation process using mercury is the only 6Li enrichment technology in practical use overseas; however, because mercury is toxic, this method cannot be industrialized in Japan. Other methods have very low separation efficiencies and are unfit for mass production. Because it is difficult to import 6Li from overseas, the establishment of a 6Li enrichment technology that is unique to Japan is an issue of top priority for the realization of fusion reactors.
Therefore, we have proposed a new and original process that uses an ionic liquid with electrodialysis, thereby establishing an innovative Li isotope separation technology. The new approach demonstrates excellent environmental resistance, is amenable to mass production, and is highly energy efficient (Fig.4-24, Fig.4-25). Lithium ions move by electrodialysis between the cathode and the anode in lithium solutions. Because the ionic mobility of 6Li ions is higher than that of 7Li ions, 6Li can be enriched on the cathode side of a cell.
Using PP13-TFSI (C11H20OF6N2O4S2) as the ionic liquid, we obtained a maximum of 1.4 for the 6Li isotope separation coefficient (Fig.4-25, top). Furthermore, we found that it is possible to obtain the separation efficiency for the 6Li isotope that ranges from 1.05 to 1.15 (Fig.4-25, bottom). These results show that the 6Li isotope separation coefficient of this method is the same as or better than that of the amalgamation process using mercury (1.06).
Currently, Li recovery technology from seawater and used batteries is developing this technology, which is sponsored by the Funding Program for Next Generation World-Leading Researchers (NEXT Program) in the Cabinet Office, Government of Japan. In addition, this technology has become a focus of great attention by the electric vehicle industry, which depends on Li ion batteries.
The present study was sponsored by the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).