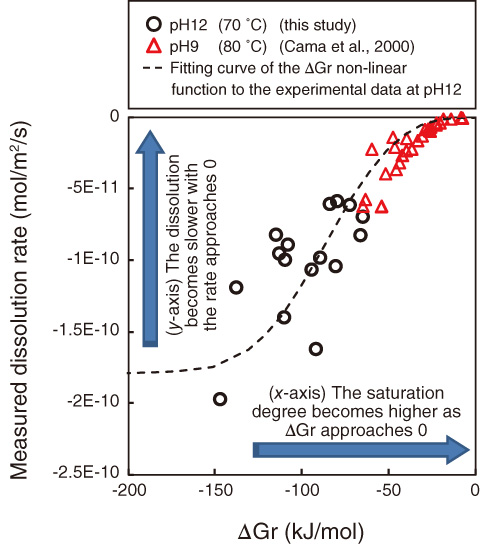
Fig.3-24 Smectite dissolution rate as a function of the degree of saturation
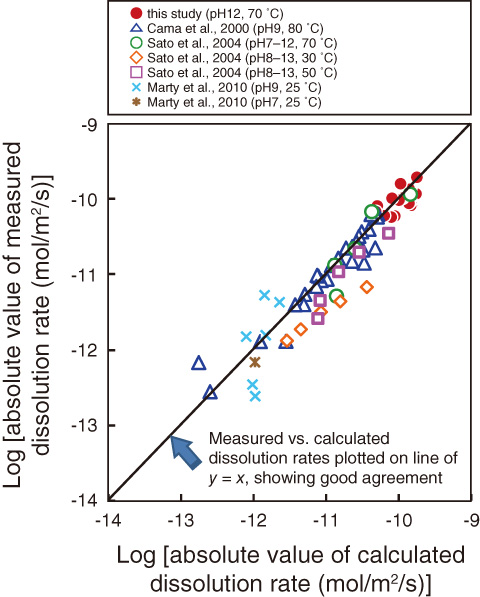
Fig.3-25 Comparison of calculated and experimentally measured smectite dissolution rates
Smectite-rich bentonites have a number of attractive physicochemical properties that make them suitable for use as a buffer material in the geological disposal of transuranic (TRU) wastes. Cementitious materials, used for their familiar engineering properties, can, however, generate high alkaline conditions of pH ≧ 12.5. Such a high pH can potentially cause alteration of bentonite, including the dissolution of smectite, and consequently the loss of the attractive physicochemical properties (e.g., the osmotic swelling capacity and consequently the plasticity and impermeability) of the bentonite buffer. The dissolution of smectite is, therefore, a key process in the evaluation of the long-term evolution of the bentonite buffer.
In this study, laboratory experiments were conducted to establish the smectite dissolution behavior under high alkaline conditions, and a dissolution rate equation for smectite was derived. A novel method using atomic force microscopy developed by Hokkaido University and Kanazawa University was used to observe the change in smectite particle size during dissolution. On the basis of the observed changes in particle size, an average dissolution rate for each experiment was determined statistically. The dissolution rates of smectite at pH12 were found to decrease with an increase in the degree of saturation (Fig.3-24). By combining these results with other published rate data obtained at pH9, a rate equation describing the dependence of smectite dissolution on the pH, temperature, and degree of saturation was determined. The smectite dissolution rates predicted by the rate equation are in good agreement with experimentally measured values at pH7-13 and 25-80 °C under a range of saturation conditions (Fig.3-25). The rate equation can therefore be used to describe the smectite dissolution rates in the bentonite buffer in response to changes in the chemical conditions expected over the temporal and spatial scales of geological disposal of TRU wastes.
Part of this study was conducted under a contract with the Hokkaido University.
<Previous: 3-7 | Next: 4 Nuclear Fusion Research and Development >