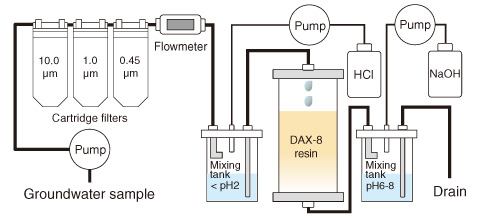
Fig.3-21 Sequential extraction system used for isolation of groundwater humic substances (HSs)
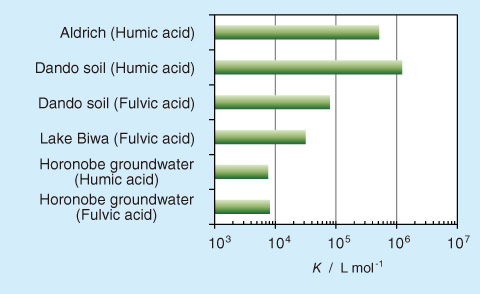
Fig.3-22 Conditional binding constants (K) of Eu3+ to groundwater HSs and comparison with those of HSs from surface environments in Japan
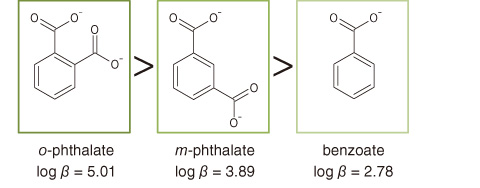
Fig.3-23 Magnitude relation between the stability constants (β) of Eu3+ to simple carboxylic acids and their structural features
Humic substances (HSs) are a type of natural organic matter that is ubiquitous in the environment. They can form soluble and stable complexes with metal ions. Because HSs are known to be dissolved even in deep groundwater, their effect on facilitating the migration of radionuclides in underground environments is a major concern for safety assessment of geological disposal systems for high-level radioactive waste.
Previous works in other countries have shown that deep groundwater HSs have the same degree of affinity for binding radionuclides as HSs from surface environments. However, it is not always true that such findings can be extended to all deep groundwater HSs because the structural feature of HSs can depend strongly on their origin.
In this study, HSs were isolated from deep groundwater at the Horonobe Underground Research Laboratory site (Fig.3-21), and the conditional binding constants (K) of Eu3+ for the groundwater HSs were then evaluated by a spectroscopic method. It was found that the groundwater HSs had the smallest K value among the HSs examined, which were two orders of magnitude smaller than those of the humic acid in the Aldrich and Dando soils (Fig.3-22). On the basis of the relationship between the stability constants (β) of Eu3+ to the simple carboxylic acids and their structural features (Fig.3-23), the low affinity for Eu3+ might be due to the fact that the carboxylate groups in the humic molecule are separated by a considerable distance.
These results demonstrate that some deep groundwater HSs in Japan have binding affinities that differ from those of HSs obtained from surface environments. In addition, our results show that the existing model and database, which were developed for HSs from surface environments, cannot be useful for deep groundwater HSs. Thus, these findings suggest that a model and database for deep groundwater HSs should be developed for realistic modeling.
The present study was partially funded by the Ministry of Economy, Trade and Industry (METI).